December 9, 2015 weblog
Multi-sponge dressing when each second counts in trauma scene
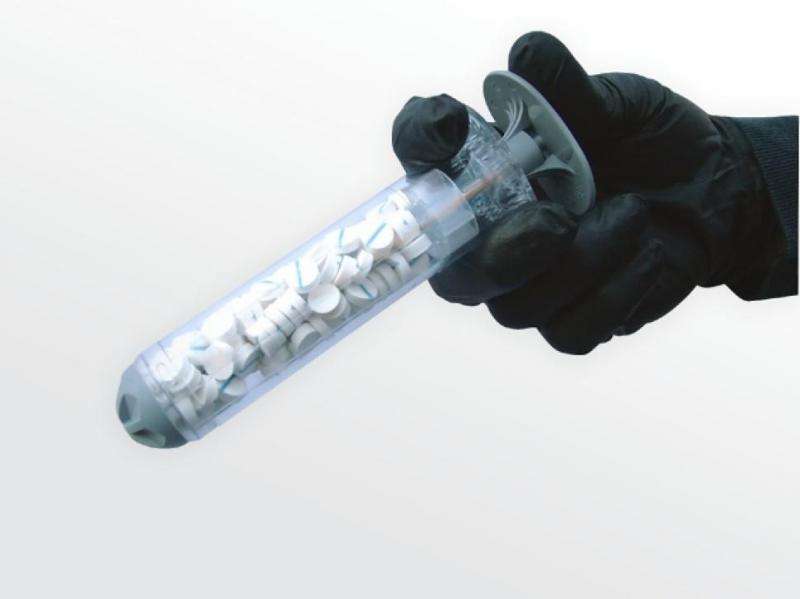
(Medical Xpress)—The US Food and Drug Administration has given the green light for XSTAT 30, a multi-sponge dressing for life-threatening bleeding from wounds in areas that a tourniquet cannot do in battlefield and civilian settings.
"When a product is developed for use in the battlefield, it is generally intended to work in a worst¬case scenario where advanced care might not be immediately available," said William Maisel, M.D., M.P.H. He is acting director of the Office of Device Evaluation in the FDA's Center for Devices and Radiological Health.
Now the product can support life-threatening trauma scenes to help first responders in civilian settings, when care at an emergency care facility cannot be achieved in minutes. Early control of severe bleeding may prevent shock and may be life-saving, said the FDA.
The dressing can be used for up to four hours, which could allow time for the patient to receive surgical care.
"According to the United States Army Institute of Surgical Research," said the FDA, "30 to 40 percent of civilian deaths by traumatic injury are the result of hemorrhaging. Of those deaths, 33 to 56 percent occur before the patient reaches a hospital."
How does it work? The FDA clearance announcement on December 7 said the device comes in packages of one or three syringe-style applicators containing 92 compressed, cellulose sponges with absorbent coatings. "The sponges expand and swell to fill the wound cavity, creating a temporary physical barrier to blood flow. The number of sponges needed for effective hemorrhage control will vary, depending on the size and depth of the wound. Each applicator can absorb about a pint of blood, and up to three applicators may be used on a patient."
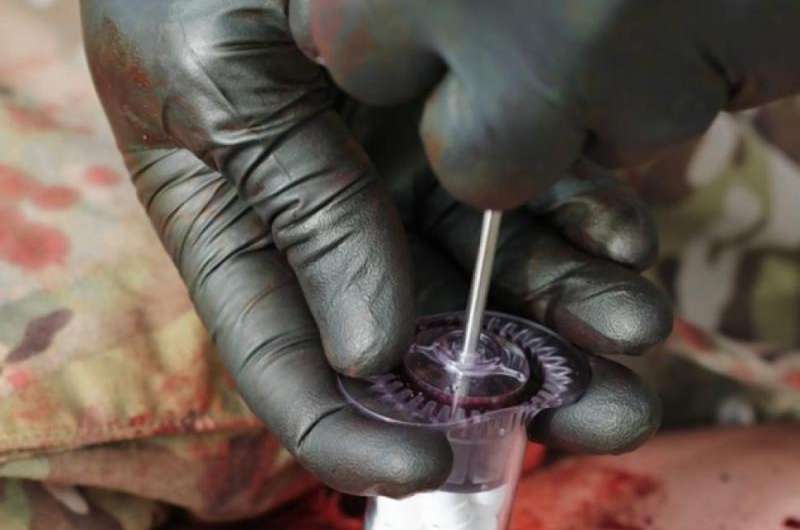
Oregon-based RevMedx is the company behind XSTAT 30; the company is focused on devices designed for combat medics and civilian first responders.
In its product description of XSTAT 30, it stated that it is not indicated for use in: the thorax; the pleural cavity; the mediastinum; the abdomen; the retroperitoneal space; the sacral space above the inguinal ligament; or tissues above the clavicle. The company also said "It should only be used for patients at high risk for immediate life-threatening bleeding from, hemodynamically significant (Advanced Trauma Life Support class 3 or 4 hemorrhagic shock), non-compressible junctional wounds, and when definitive care at an emergency care facility cannot be achieved within minutes."
The FDA cleared XSTAT 30 through the 510(k) review process after the manufacturer demonstrated the product was substantially equivalent to the XSTAT, which was granted marketing authorization for battlefield use in April 2014.
George Dvorsky in Gizmodo said, "Guns in the United States kill about 33,000 people annually, of which some 20,000 are suicides and 11,000 are homicides. This year alone, the United States has experienced 462 mass shootings."
More information: www.fda.gov/NewsEvents/Newsroo … ements/ucm475810.htm
© 2015 Medical Xpress














