Carnegie Mellon joins IARPA project to reverse-engineer brain algorithms
Carnegie Mellon University is embarking on a five-year, $12 million research effort to reverse-engineer the brain, seeking to unlock the secrets of neural circuitry and the brain's learning methods. Researchers will use these insights to make computers think more like humans.
The research project, led by Tai Sing Lee, professor in the Computer Science Department and the Center for the Neural Basis of Cognition (CNBC), is funded by the Intelligence Advanced Research Projects Activity (IARPA) through its Machine Intelligence from Cortical Networks (MICrONS) research program. MICrONS is advancing President Barack Obama's BRAIN Initiative to revolutionize the understanding of the human brain.
"MICrONS is similar in design and scope to the Human Genome Project, which first sequenced and mapped all human genes," Lee said. "Its impact will likely be long-lasting and promises to be a game changer in neuroscience and artificial intelligence."
Lee will work with co-principal investigators Sandra Kuhlman, assistant professor of biological sciences at Carnegie Mellon and the CNBC, and Alan Yuille, the Bloomberg Distinguished Professor of Cognitive Science and Computer Science at Johns Hopkins University, to discover the principles and rules the brain's visual system uses to process information. This deeper understanding could serve as a springboard to revolutionize machine learning algorithms and computer vision.
In particular, the researchers seek to improve the performance of neural networks—computational models for artificial intelligence inspired by the central nervous systems of animals. Interest in "neural nets," which initially peaked in the 1990s, has recently undergone a resurgence thanks to growing computational power and datasets. Neural nets now are used in a wide variety of applications in which computers can learn to recognize faces, understand speech and handwriting, make decisions for self-driving cars, perform automated trading and detect financial fraud.
"But today's neural nets use algorithms that were essentially developed in the early 1980s," Lee said. "Powerful as they are, they still aren't nearly as efficient or powerful as those used by the human brain. For instance, to learn to recognize an object, a computer might need to be shown thousands of labeled examples and taught in a supervised manner, while a person would require only a handful and might not need supervision."
Artificial neural nets process information in one direction, from input nodes to output nodes. But the brain likely works in quite a different way. Neurons in the brain are highly interconnected, suggesting possible feedback loops at each processing step. What these connections are doing computationally is a mystery; solving that mystery could enable the design of more capable neural nets.
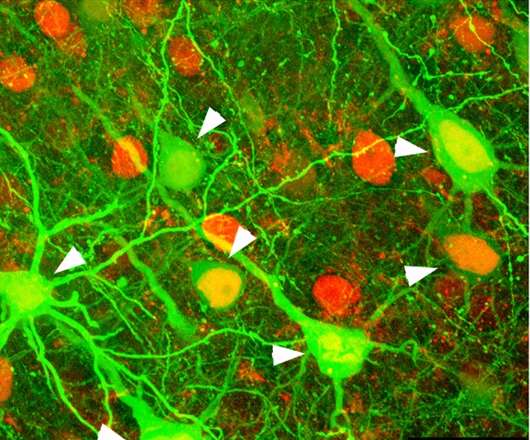
To better understand these connections, Kuhlman will use a technique called "2-photon calcium imaging microscopy" to record signaling of tens of thousands of individual neurons in mice as they process visual information, an unprecedented feat. In the past, only a single neuron, or tens of neurons, typically have been sampled in an experiment, she noted.
"By incorporating molecular sensors to monitor neural activity in combination with sophisticated optical methods, it is now possible to simultaneously track the neural dynamics of most, if not all, of the neurons within a brain region," Kuhlman said. "As a result we will produce a massive dataset that will give us a detailed picture of how neurons in one region of the visual cortex behave."
Meanwhile, the CMU-led team will collaborate with another MICrONS team at the Wyss Institute for Biologically Inspired Engineering, led by George Church, professor of genetics at Harvard Medical School. The Harvard-led team, working with investigators at Cold Spring Harbor Laboratory, MIT and Columbia University, is developing revolutionary techniques to reconstruct the complete circuitry of the neurons recorded at CMU. The database, along with two other databases contributed by other MICrONS teams, unprecedented in scale, will be made publicly available for research groups all over the world.
In this MICrONS project, CMU researchers and their collaborators in other universities will use these massive databases to evaluate a number of computational and learning models as they improve their understanding of the brain's computational principles and reverse-engineer the data to build better computer algorithms for learning and pattern recognition.
"The hope is that this knowledge will lead to the development of a new generation of machine learning algorithms that will allow AI machines to learn without supervision and from a few examples, which are hallmarks of human intelligence," Lee said.
"Extracting the brain's secret algorithms in learning and inference from this massive amount of data to advance machine learning is extremely ambitious and might be the most uncertain part of this project," said Andrew Moore, dean of CMU's School of Computer Science. "It's the equivalent of a moonshot, but CMU is one of the world's best places to do this, because we have a very strong tradition and community in artificial intelligence. We also have a strong community of theoretical and experimental neuroscientists working with the Center for the Neural Basis of Cognition and the university's BrainHub initiative."
The CNBC is a collaborative center between Carnegie Mellon and the University of Pittsburgh. BrainHubSM is a neuroscience research initiative that brings together the university's strengths in biology, computer science, psychology, statistics and engineering to foster research on understanding how the structure and activity of the brain give rise to complex behaviors.
In addition to Kuhlman and Yuille, the MICrONS team includes Abhinav Gupta, assistant professor of robotics; Gary Miller, professor of computer science; Rob Kass, professor of statistics and machine learning and interim co-director of the CNBC; Byron Yu, associate professor of electrical and computer engineering and biomedical engineering and the CNBC; and Steve Chase, assistant professor of biomedical engineering and the CNBC. Another member is Ruslan Salakhutdinov, one of the co-creators of the deep belief network, a new model of machine learning that was inspired by recurrent connections in the brain. Salakhutdinov will join CMU as an assistant professor of machine learning in the fall.
Other members of the team include Brent Doiron, associate professor of mathematics at Pitt, and Spencer Smith, assistant professor of neuroscience and neuro-engineering at the University of North Carolina.