What viruses do to neuronal stem cells—effects of congenital transmission
Congenital transmission (from mother to unborn child) of viruses can cause abnormal brain development in the fetus. Examples of viruses that can pass through the placenta and into the fetal brain include cytomegalovirus, rubella, and zika virus. A study published on April 14th in PLOS Pathogens examines the effects of human cytomegalovirus (HCMV) infection on neuronal stem cells and reports that the virus delays or prevents proper differentiation of the stem cells into mature brain cells by activating a key signaling pathway.
About 1 % of newborns in the US are congenitally infected with HCMV. The majority of them show no symptoms, but about 20% have neurological problems that are obvious either at birth or develop soon thereafter. The most severe cases show brain developmental abnormalities such as microcephaly.
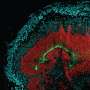
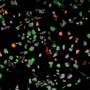
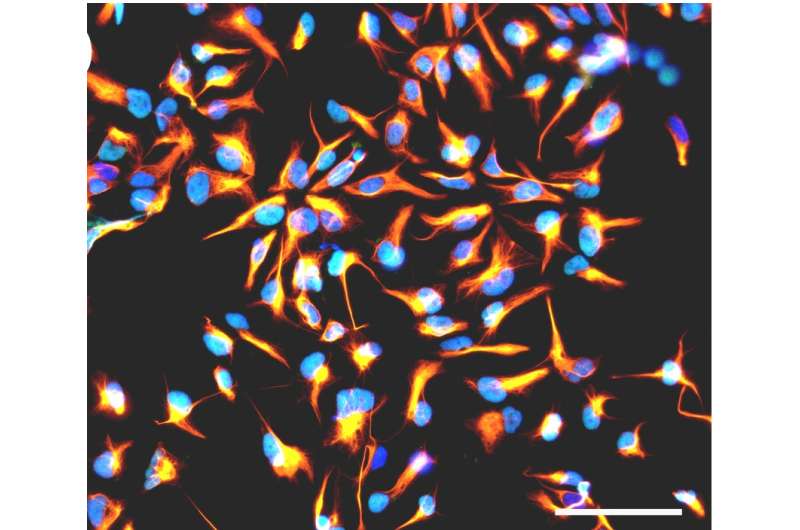
To study how viral infection affects brain development, Stéphane Chavanas, from the Université de Toulouse and INSERM UMR1043, in France, and colleagues developed a new model of infection based on human neural stem cells (NSCs) that normally produce neurons (nerve cells) at a high frequency. They found that HCMV infection substantially reduced the rate of neurons generated by the NSCs.
To get at possible mechanisms, the researchers studied the outcomes of infection on Peroxisome Proliferator-Activated Receptor gamma (PPARg), a transcription factor critical in the developing brain. HCMV infection dramatically increased PPARg levels and activity. Consistent with these findings, levels of 9-hydroxyoctadecadienoic acid (9-HODE), a known PPARg activator, were significantly increased in infected NSCs compared with uninfected ones.
Exposure of uninfected NSCs to 9-HODE recapitulated the effect of infection on PPARg activity. Consistent with this, both pharmacological activation of PPARg in uninfected NSCs or treatment of uninfected NSCs with 9-HODE was sufficient to impair neuron production. Moreover, treatment of HCMV infected NSCs with a drug that inhibits PPARg restored a normal rate of neuron production.
To assess the pathophysiological relevance of the experiments in NSCs, the researchers investigated the expression of PPARg in 20 brain samples from aborted fetuses with congenital HCMV infection and 4 samples from uninfected control fetuses. PPARg, they found, was present in the nuclei of cells (where it is active) in brain regions that are normally characterized by active neuron production in infected brain samples, but not in samples from uninfected fetuses.
"NSCs", the researchers state, "turned out to be an invaluable tool for modeling functional correlates of HCMV infection, and this cell platform may probably be extended to other viral pathologies of the central nervous system", such as congenital infection by zika virus. They conclude that their findings "reveal a key role for PPARg in neurogenesis and in the pathophysiology of HCMV congenital infection" and "pave the way to the identification of PPARg gene targets in the infected brain".
More information: Rolland M, Li X, Sellier Y, Martin H, Perez-Berezo T, Rauwel B, et al. (2016) PPARγ Is Activated during Congenital Cytomegalovirus Infection and Inhibits Neuronogenesis from Human Neural Stem Cells. PLoS Pathog 12(4): e1005547. DOI: 10.1371/journal.ppat.1005547