'Inexpensive old drug' may prevent birth damage in high-risk newborns, study shows
A 27-year-old drug for anemia may protect newborns at high risk for brain damage, according to the results of a multisite trial led by researchers at UC San Francisco.
Each year more than 800,000 deaths worldwide and many thousands of cases of permanent brain damage in the U.S. are attributed to hypoxic-ischemic encephalopathy (HIE), a dysfunction of the nervous system caused by birth complications resulting in a drop in oxygen supply and inadequate blood flow to the brain and other organs.
Standard of care for HIE is hypothermia in which the head or whole body is cooled to 33.5ºC (92.3ºF) in order to accelerate healing. But hypothermia doesn't save all patients.
"More than 40 percent of infants will die or suffer moderate to severe disabilities, including cerebral palsy, intellectual impairment and epilepsy," said Yvonne Wu, a child neurologist and professor of Neurology and Pediatrics at UCSF Benioff Children's Hospital San Francisco, and lead author of the study. "We wanted to find something that could amplify effectiveness."
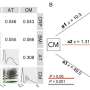
In a phase II trial conducted at seven hospitals throughout the nation, the researchers compared outcomes in 26 full-term newborns with HIE, who were treated with hypothermia and placebo, to 24 who were treated with both hypothermia and five infusions of EPO, or erythropoietin, a man-made version of a natural hormone that stimulates the production of red blood cells.
MRIs Show Fewer Incidences of Brain Injury
The drug was approved by the Food and Drug Administration in 1989 to treat anemia in patients with chronic renal failure. It was later prescribed for children and infants. Previous studies have shown that EPO is an anti-inflammatory and fights apoptosis or cell death. Additionally, it has been demonstrated to promote the development of nervous tissue and tissue remodeling after oxygen deprivation.
When the MRIs of the treated newborns were examined at an average 5 days of age, eight in the EPO group (33.3 percent) were found to have no brain injury, versus three in the placebo group (11.5 percent). One infant in the EPO group had a severe or moderate injury compared with 11 such injuries in the placebo group.
The findings appear in the May 3, 2016, issue of the journal Pediatrics.
The researchers conducted testing at 12 months of age and found that the EPO-treated group developed superior motor skills compared to the non-EPO treated cohort, but these results are preliminary and need to be confirmed, Wu cautioned.
"There is a large range of normal development at age 1. Some children are walking independently while others won't achieve this for weeks or months. As the children grow older, testing of motor, language and social cognition will become more meaningful," she said.
Two deaths occurred in the EPO-treated newborns, versus five in the placebo group – a difference that was not clinically significant due to the low number of infants in the trial. Serious adverse effects were reported in nine newborns, but were evenly distributed in both groups and none were attributed to EPO, said Wu.
'Strong Suggestion that Patients are Doing Better'
"It is clear that this therapy is safe as used in this study and there is a strong suggestion that the patients are doing better than would be expected long term," said senior author Roberta Ballard, MD, a neonatologist and professor of Pediatrics at UCSF Benioff Children's Hospital San Francisco. "It would be very encouraging if we find that an inexpensive old drug, costing $60 per infusion, rather than a new drug that costs more than $2 billion to develop may prevent untold suffering. A larger trial will allow a definitive answer to whether there should be a change in clinical care for this devastating condition."
HIE occurs in 1 to 3 per 1,000 full-term births. It accounts for 22 percent of annual neonatal deaths worldwide, totaling 814,000 deaths in 2008, according to the Child Health Epidemiology Reference Group of the World Health Organization and the United Nations Children's Fund.
"We're hopeful that EPO not only reduces the extent of brain injury, but also allows the brain to be more effective at repairing itself during the recovery process," Wu said. "While research with more participants is needed to determine whether EPO saves lives, we are heartened by the fact that among those infants that survived, the degree of brain injury was generally less severe than in the placebo group."
The study was supported by funding from the Thrasher Research Fund. The researchers had no financial conflicts to report.