Study confirms long-term benefit of anti-VEGF therapy for age-related macular degeneration
Patients with age-related macular degeneration (AMD), the most common cause of major vision loss in older people, still show benefits from a new class of therapy—originally developed to treat cancer—after long-term treatment.
The finding comes from a follow-up study of patients in a large clinical trial who were treated with drugs that inhibit vascular endothelial growth factor (VEGF). After an average of 5.5 years from starting treatment, half the patients retained visual acuity in the affected eye of 20/40 or better, which is normally sufficient for driving without glasses. Before anti-VEGF drugs became available a decade ago, AMD patients fared much worse.
The study, coordinated by a team at the Perelman School of Medicine at the University of Pennsylvania, confirms the long-term clinical value of targeting VEGF, a molecule whose abnormal overproduction in the eye drives vision loss in AMD. Patients in the study took Avastin (bevacizumab) or Lucentis (ranibizumab), the first two widely used anti-VEGF drugs.
"The good news is that patients are having much better visual outcomes than even dreamed about ten years ago—but there's still a considerable proportion of patients for whom long term outcomes are not good, and we need better treatments for them," said senior investigator Maureen G. Maguire, PhD, Carolyn F. Jones Professor of Ophthalmology at Penn Medicine.
The results are being presented today at the annual meeting of the Association for Research in Vision and Ophthalmology (ARVO) in Seattle and published simultaneously in the journal Ophthalmology.
AMD before anti-VEGF therapies
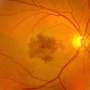
AMD in all its forms is estimated to affect about 6.5 percent of people in the U.S. over age 40, or roughly ten million Americans. It features the degeneration of the macula, the central region of the retina, in which a concentration of color-sensing cone cells normally provides high-resolution color vision wherever the gaze is directed.
The most severe AMD-related vision loss comes from "wet" or "neovascular" AMD, in which new blood vessels grow under or into the retina. The resulting vessels, that depend on VEGF, are abnormally fragile and leaky, and their proliferation and associated fluid buildup lead to macular damage and increasing loss of vision in the central visual field, making it increasingly difficult for sufferers to drive, read, and recognize faces. Left untreated, patients can lose most of their central vision in their eye within 1 to 2 years and about half will lose vision in their second eye within another 5 years.
Before anti-VEGF treatments were available, ophthalmologists used light-activated (photodynamic) drugs to block and destroy the abnormal vessels. This approach had a small impact on the course of disease, but in the 1990s, researchers discovered the link between wet AMD and VEGF, and in 2004, the first anti-VEGF drug for treating wet AMD received FDA approval.
Lucentis, Avastin and CATT
In 2006, the FDA approved a more effective anti-VEGF treatment for wet AMD, Lucentis (ranibizumab), a fragment of an antibody that binds to the VEGF protein. Around this time, ophthalmologists also began to treat wet AMD with a closely related anti-VEGF antibody fragment, Avastin (bevacizumab), which was FDA-approved for treating cancers but is much cheaper than Lucentis at the doses used for AMD therapy.
In 2008, Maguire and colleagues at Penn Medicine, along with Cleveland Clinic researchers led by Daniel F. Martin, MD, and researchers from other institutions, set up a large clinical trial, CATT (Comparison of AMD Treatments Trials) to compare the two anti-VEGF therapies. Working with almost 900 collaborators at dozens of eye clinics across the nation, with sponsorship from the National Eye Institute, they found that Avastin and Lucentis have essentially the same effectiveness and safety after one and two years of treatment.
In the new study, the CATT Follow-up Study, Maguire and colleagues examined visual acuity and other outcomes in CATT patients who continued to receive treatment after the end of the two-year trial. During this follow-up period, which averaged 3.5 years, most patients continued to be treated with either Avastin or Lucentis, although some switched from one to the other. Thus the study was meant not to compare the drugs again but merely to gauge the longer-term collective impact of these anti-VEGF therapies.
Of the 647 patients for whom visual acuity measurements were available during the follow-up period, 321 (49.6 percent) had at least 20/40 vision in the affected eye—vision that allows driving or reading a newspaper. By contrast, prior studies have found that at two years after diagnosis, fewer than 10 percent of untreated wet AMD eyes retain 20/40 vision, and fewer than 15 percent retain 20/40 vision if treated with photodynamic therapy.
Even so, the CATT patients did experience some vision loss during the follow-up period. The nearly 50 percent retention of 20/40 vision was a drop from nearly 70 percent at the end of the two-year trial. Moreover, the percentage of eyes with 20/200 vision (commonly considered "legally blind") or worse jumped to 20 percent during the follow-up period, from just 5 percent at two years. Measurements of retinal thickness and imaging of the back of the eye and its vessels, which were available for more than 500 patients during the follow-up period, also revealed signs of modestly worsening disease.
"When we first treat neovascular AMD patients, after they've noticed some initial vision loss, they typically gain about two lines worth of visual acuity on the eye chart, and what we've seen in this study is that between year two and about year five of anti-VEGF treatment they lose most of that improvement," said Maguire. "On the other hand, if they had been untreated they never would have seen any improvement at all—their vision would have gotten worse and worse."
Future advances in AMD therapy are likely to come in part from anti-VEGF treatments that are more effective and/or less burdensome to administer. Avastin and Lucentis are meant to be administered by injection into the eye at a clinic up to every four weeks. A newer anti-VEGF therapy, Eylea (aflibercept) has been shown to work well with injections every eight weeks. "Ideally what we'd like is a treatment that doesn't require the patient to come back for evaluation until six to twelve months later," said Maguire.
Targeting factors other than VEGF, she added, will also probably be necessary to provide a more comprehensive prevention of AMD disease processes, including those that do not directly involve new blood vessel growth.
The CATT Research Group's writing committee, which prepared the follow-up study for publication, included Maguire, head of the CATT coordinating center at Penn; Martin, the chair of the research group; Gui-shuang Ying, PhD, of the department of Ophthalmology at Penn; Juan E. Grunwald, MD, principal investigator at the Fundus Photograph Reading Center at Penn; Ebenezer Daniel, MBBS, PhD, also of the Fundus Photograph Reading Center; Cynthia A. Toth, MD, of Duke University School of Medicine; Frederick L. Ferris III, MD of the National Eye Institute; and Stuart L. Fine, MD, formerly chair of the Penn department of Ophthalmology and now at the University of Colorado.