ECDC rapid risk assessment outlines actions to reduce the spread of the mcr-1 gene
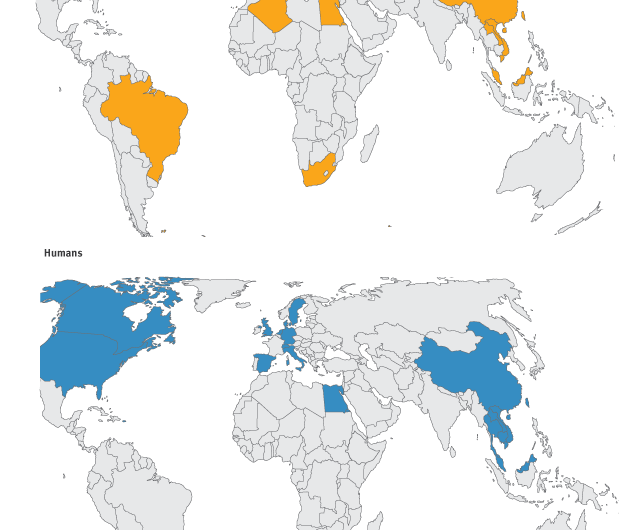
The recently recognised global distribution of the mcr-1 gene poses a substantial public health risk to the EU/EEA. The gene is widespread in several continents and has been detected in bacteria isolated from multiple different sources such as food-producing animals, food, the environment and humans.
This new mechanism of resistance to colistin is of exceptional public health concern because it further limits treatment options in patients with infections caused by multidrug-resistant (MDR) Gram-negative bacteria, and also because it is a highly mobile type of drug resistance (the gene is on a plasmid) that can spread more easily between bacteria.
MDR Gram-negative bacteria, including carbapenem-resistant Enterobacteriaceae strains that acquire the mcr-1 gene, remain susceptible to only a few antimicrobial agents, which means that infections caused by these strains are very difficult to treat and result in excess mortality. As the development of new antimicrobials is unlikely to provide a solution anytime soon, it is crucial to take measures to control the spread of mcr-1 and thus protect the activity of colistin.
Although there are still major information gaps, relating to the current prevalence of colistin resistance in human clinical isolates in the EU/EEA, as well as to the historical and current prevalence of colistin resistance due to the mcr-1 gene, the issue of further spread of mcr-1 needs to be taken seriously and be carefully monitored by EU/EEA countries.
Andrea Ammon, ECDC Acting Director, said: "the spread of the mcr-1 gene represents another step towards pandrug resistance and infections that would be very difficult, if not impossible, to treat. Further spread would increase the morbidity and mortality in patients undergoing advanced medical procedures, thus having a profound effect on the practice of medicine."
In its rapid risk assessment, ECDC outlines a number of actions that need to be considered to reduce identified risks of mcr-1 spread. These include improved laboratory methods for colistin resistance testing and mcr-1 detection, improved surveillance, options for appropriate clinical management, and actions to prevent transmission in healthcare settings as well as in the community.
















