Dual antigen targeting may improve CAR T cell cancer therapy
A solid tumor comprises a heterogeneous population of cells that differ from each other on their type of surface molecules. In an article published today in the Journal of Clinical Investigation, researchers from Baylor College of Medicine, Texas Children's Cancer Center and the Center for Cell and Gene Therapy at Baylor, Texas Children's Hospital and Houston Methodist Hospital show a new strategy, based on targeting specific surface molecules, that eliminates most of the cancer cells in a mouse model of glioblastoma, mitigates tumor escape, controls tumors better and improves animal survival.
"Tumors are a community of heterogeneous cells that use this heterogenicity to their advantage," said senior author Dr. Nabil Ahmed, associate professor of pediatrics at Baylor and Texas Children's Cancer Center.
The researchers wanted to understand what happens to tumors when a treatment targets a single surface molecule. First-author Dr. Meenakshi Hegde, assistant professor in pediatrics at Baylor, worked with glioblastoma tumor cells in the lab and in a mouse model of glioblastoma. She targeted a single surface molecule on the tumor cells with chimeric antigen receptor T cells, or CAR T cells. CAR T cells are T cells – a type of immune cells involved in defense against tumors – that have been programmed to recognize and kill tumor cells carrying one specific antigen on the surface of cancer cells through an artificial molecule expressed on their surface, the CAR.
Hegde asked, when we target a single antigen on tumor cells with CAR T cells, what happens to the tumor? CAR T cells eliminated the cells that had the target on their surface, the one they had been programmed to recognize, leaving behind the cells without the target. When Hegde carried out this experiment in the mouse model, she observed that, although part of the tumor had been eliminated by CAR T cells, in time, the tumor relapsed. The relapsed tumor cells were not carrying the target the CAR T cells recognized but instead carried other surface molecules.
These results taught the researchers that "targeting one type of surface molecule on a tumor kills only the cells that carry that antigen. The cells that do not carry the surface molecule are spared and can continue growing. They become the cells that cause relapse," said Ahmed. The researchers then tested what would happen if they used CAR T cells to target two different tumor surface molecules, simultaneously. They found that "if we target more than one antigen, we have a better chance of mitigating tumor escape," said Ahmed.
The antigens the researchers selected, called HER2 and IL13Rα2, are good candidates for tumor therapy because they are expressed on the surface of most glioblastoma cells, but expressed in very low quantities on normal tissues of the body. This is important to minimize CAR T cells targeting and killing normal cells.
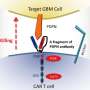
The researchers determined what would happen if the CARs on T cells that bound to HER2 and IL13Rα2 were single bivalent molecules; that is, one molecule that could bind both antigens simultaneously. When T cells recognized HER2 and IL13Rα2 simultaneously through a single bivalent CAR molecule, the anti-tumor "activity was much higher than when separate CAR molecules were used," said Ahmed.
The effect of CAR T cells recognizing HER2 and IL13Rα2 simultaneously was more than the addition of the effects of individual recognition of the molecules. "The activity is super additive," said Ahmed.
Then, Hegde and Ahmed wanted to visualize what was happening between bivalent CAR T cells and glioblastoma cells.
"First, we tried to answer the question with macromolecular imaging, so we teamed up with Dr. Matthew Baker, assistant professor of biochemistry and molecular biology at Baylor and the National Center for Macromolecular Imaging in Houston," said Ahmed. "With this technology, we simulated fitting the molecules together – HER2 and IL13Rα2 with the bivalent CARs that targeted them simultaneously. We determined that the energy was favorable for this three-way fit, the bivalent CAR molecule and both HER2 and IL13Rα2. The CAR could perhaps bind both target molecules in tandem. We thus called it a Tandem CAR, or TanCAR."
A natural next step was to try to visualize what actually happened between TanCAR T cells and glioblastoma cells when they interacted with one another. "We went to the Center for Human Immunobiology, at Texas Children's Hospital, where director Dr. Jordan Orange of Baylor and colleagues have advanced expertise on the imaging of immunological synapses," said Ahmed. Orange, professor of pediatrics – rhematology, and Dr. Malini Mukherjee, research associate of pediatrics – human immunobiology, used a new technology called Stimulation Emission Depletion (STED) Microscopy, which brings down the resolution to less than 150 nanometers, close to detecting a single molecule. This was the first time such technology was used to visualize CAR immune synapses.
"With STED we were amazed to see that when TanCAR T cells and glioblastoma cells touch each other, they make a concentrated disc of the surface molecules, an immunological synapse. Once CARs bring the glioblastoma surface antigens together, this triggers activation inside the T cells that leads to the death of glioblastoma cells. Simply bringing HER2 and IL13Rα2 together leads the cancer cells to their demise," said Ahmed.
The researchers anticipate that treating solid tumors with TanCAR T cells can have promising results in clinical trials. "When we target a single entity in a tumor, the tumor finds a way to continue living without it. But when we target two entities simultaneously with TanCAR T cells, we enable the T cells to kill tumor cells more specifically and effectively. Two is better than one," said Ahmed.
More information: Meenakshi Hegde et al, Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape, Journal of Clinical Investigation (2016). DOI: 10.1172/JCI83416