Engineering a cardiac stem cell therapy inspired by the body itself
During a heart attack, known clinically as acute myocardial infarction (MI), cardiac muscle cells are severely damaged and eventually die.
According to the American Heart Association, MI is the leading cause of death globally. This is due, in part, to the human heart's limited capacity to repair or regenerate its cells, known as cardiomyocytes. In the past decade, stem cell therapy has been explored as a way to regenerate cardiomyocytes, but with mixed results.
Ohio State researchers, led by Biomedical Engineering Professor Xiaoming He, have invented a new stem cell therapy method inspired by nature that is showing great promise. In study results published in the current issue of Nature Communications, their method significantly improves the survival of implanted stem cells compared with conventional approaches.
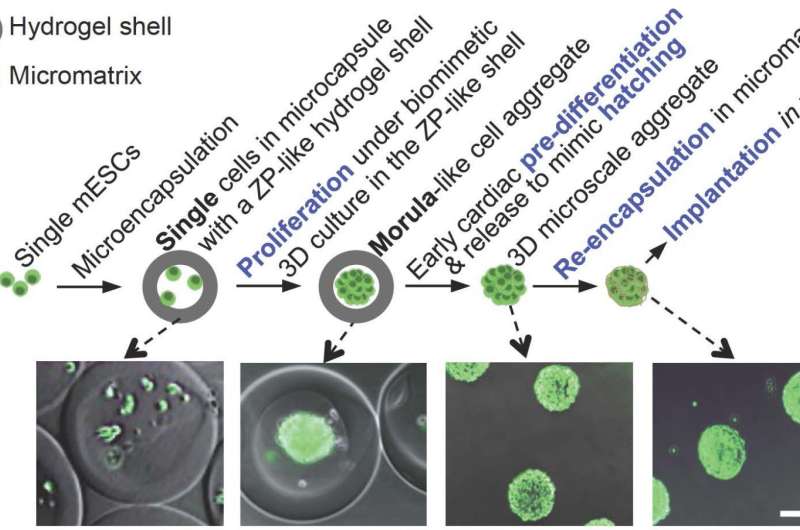
The researchers' preparation of pluripotent stem cells for implantation into the heart is similar to how the female body prepares embryonic cells for implantation into the uterus wall during reproduction, namely pre-differentiation and two phases of encapsulation. Pluripotent stem cells are able to make almost any cell or tissue the body needs to repair itself.
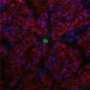
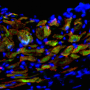
The natural reproductive steps include organized multiplication of cells to form an aggregate, or a morula, encased in a thin protective hydrogel shell. Similarly, the researchers encapsulated murine (mouse) embryonic stem cells in a small core enclosed in a semipermeable hydrogel shell, where they proliferated. This embryo-like configuration greatly helps to retain what He referred to as stemness, or the cells' ability to self-renew without spontaneous differentiation. The next step in the female reproductive system is to pre-differentiate, or program, the morula into more specific cell types. For the MI treatment study, researchers pre-differentiated the aggregated stem cells into an early cardiac stage.
According to He, the pre-differentiation of the cells to early cardiac stage is critical, as it appears to minimize a common issue in current regenerative medicine approaches—the formation of teratoma, which can develop into benign or malignant tumors.
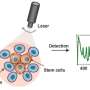
"The cells are committed to becoming cardiomyocytes," He said, "but not quite there yet." That precise timing is determined by cell biomarkers. Microarray and flow cytometry data analysis show that the aggregated cells are successfully directed into the early cardiac line following pre-differentiation.
In nature, human embryonic cells in the pre-differentiated morula—or blastocyst—hatch briefly out of the jelly-like shell and then are re-encapsulated in a new shell called a trophoblast before implantation into the uterus wall. The researchers mimicked these steps by releasing the aggregated early-stage cardiac cells and re-encapsulating them in a biocompatible and biodegradable scaffold before implantation into a damaged mouse heart.
"This micromatrix re-encapsulation procedure is opposite to that of conventional scaffold engineering where a scaffold is made first followed by seeding it with cells," He explained.
The re-encapsulation is also crucial for temporarily isolating the implanted cells from the host immune systems, so that the cells can adapt to the host microenvironment without evoking significant immune response. This ensures high survival of the cells. Following re-encapsulation in the temporary micromatrix, He and his colleagues injected the cell aggregates in mice with recent damage consistent with a heart attack.
The implanted cells demonstrated excellent capability of regenerating cardiac tissue once guided by the body's cardiac-inducive chemical, mechanical and electrical cues. The injection of encapsulated pluripotent stem cells significantly reduced fibrosis, restored cardiac function of mice with MI, and improved animal survival.
"Our bioinspired approach is based on mimicking the natural phenomena and presents multiple advantages in preparing pluripotent stem cells to treat MI and potentially other ischemic diseases," He said.
He said the next step is to conduct research on human cardiac regeneration, at which time the researchers would begin using induced pluripotent stem cells derived from human skin, fat or other tissues.
Project collaborators include Biomedical Engineering Professor Mingjun Zhang, Wexner Medical Center Pathologist and Associate Professor Rulong Shen, Wexner Medical Center Physician and College of Medicine Professor Zhenguo Liu, and College of Medicine Associate Professor Noah Weisleder, together with graduate students and postdoctoral research fellows from biomedical engineering and the Davis Heart and Lung Research Institute.
"This research is the result of an excellent combination of engineering, cell biology, physiology, pathology, and clinical medicine expertise," He said. "And at Ohio State, all of these experts are located within close proximity."
The research was partially supported by grants from National Science Foundation and National Institutes of Health.
More information: Shuting Zhao et al. Bioengineering of injectable encapsulated aggregates of pluripotent stem cells for therapy of myocardial infarction, Nature Communications (2016). DOI: 10.1038/ncomms13306