N-acetyl cysteine improves efficacy of adoptive T cell immunotherapy for melanoma
A collaborative team of investigators at the Medical University of South Carolina (MUSC) and Loyola University have demonstrated for the first time that culturing T cells in N-acetyl cysteine (NAC) before they are infused as immunotherapy improves effectiveness and outcomes in a preclinical model of melanoma. These findings were reported in the October 15, 2016 issue of Cancer Research.
Both incidence and mortality rates for metastatic melanoma continue to rise. Only about 15% of Stage IV melanoma patients receiving standard treatment can expect to survive for five years. By contrast, clinical trial data show that up to 40% of Stage IV melanoma patients survive for five years when treated with adoptive cell therapy (ACT), a form of immunotherapy that calls for infusion of autologous, melanoma-specific T cells.
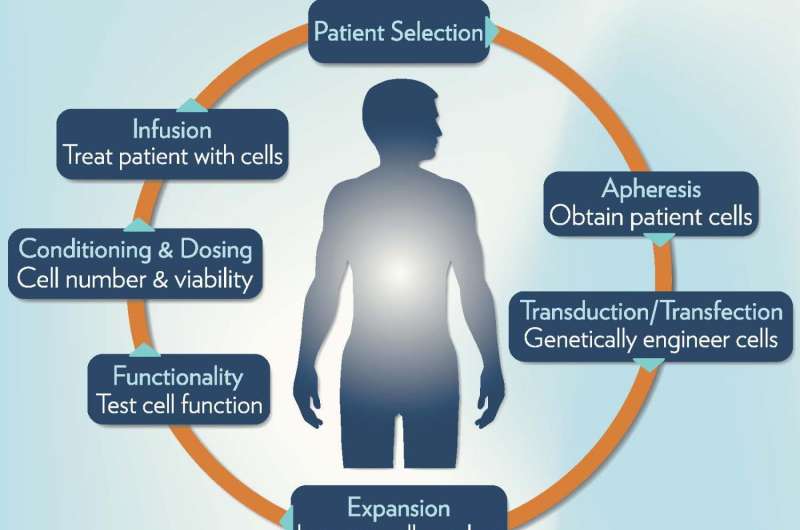
ACT aims to boost a patient's own immune responses against the cancer. To do this, the patient's own T cells are harvested, genetically modified with a therapeutic T cell receptor, activated, and then rapidly expanded to generate large numbers of T cells for therapeutic re-infusion.
Unfortunately, patient responses vary. Better outcomes are positively correlated with persistence of the transferred cells. The rapid expansion of harvested T cells before reinfusion increases their susceptibility to activation-induced cell death (AICD), prompting the authors to hypothesize that AICD reduces ACT's overall effectiveness.
Researchers have long known that factors limiting T cell persistence also limit ACT efficacy but, until now, no one knew that something as simple as changing the culture condition by supplementing with NAC could improve survival of the reinfused T cells. The research team showed that adding NAC to the in vitro T cell expansion culture prevents increases in the DNA damage marker γH2AX and significantly improves T cell persistence and immunotherapy outcomes, including reduced tumor growth and enhanced survival.
The team found that nearly 40% of NAC-cultured T cells were detectable in tumors after transfer compared to approximately 1.2% of standard-culture T cells. They also found that mice receiving NAC-cultured cells experienced significantly delayed tumor growth compared to mice receiving standard-culture cells (P<.0001).
"We were really surprised by the number of adoptively transferred T cells we saw in the tumor," said Christina Voelkel-Johnson, PhD, Associate Professor of Microbiology and Immunology at MUSC's Hollings Cancer Center and the senior author on the article. "Given the harsh environment T cells encounter within tumors, we did not expect that the number of NAC-cultured T cells would be 33-fold higher than T cells not cultured in NAC."
Adding NAC to existing protocols should pose little risk to patients, according to Voelkel-Johnson, because NAC is already in clinical use for many indications and the culturing of T cells in NAC would occur outside of the patient. "The only difference would be a change to the cell culture protocol in an effort to generate cells with an improved phenotype," said Voelkel-Johnson. Clinical trials will be needed to confirm the benefits of adding NAC to these protocols.
The road to discovering the protective role of NAC began with the MUSC/Loyola research team hypothesizing that preventing T cells from becoming susceptible to AICD during in vitro expansion might improve their persistence and effectiveness upon reinfusion.
The team started by examining the role of p53, which is crucial for coordinating cellular stress responses and determining the fates of damaged cells (i.e., whether they will be repaired or allowed to die). While it is known that inhibiting p53 protects T cells from AICD, the molecular-level mechanisms that provide this protection were unknown. The investigators created conditions similar to those that occur during in vitro T cell expansion by restimulating the T cell receptors (TCR) to assess p53 status and cell death. They found that AICD following TCR re-stimulation is accompanied by phosphorylation of p53 on Ser15 and accumulation of p53 in the cell nucleus.
The next set of experiments clarified that the PI3K-like serine kinase, ataxia telangiectasia mutated (ATM), is necessary for the phosphorylation of p53 on Ser15 after TCR restimulation. They also found that inhibiting ATM almost completely prevents cell death (99%) after TCR restimulation. Thus, ATM appears to be a required upstream factor for AICD onset.
While activation of ATM and p53 are parts of a known DNA damage response pathway, ATM also responds to oxidative stress or hypoxia. So, the team needed to determine whether the changes in ATM and p53 were being caused by DNA damage or oxidative stress/hypoxia. They looked at two established DNA damage markers, γH2AX and p-SMC-1, and found that, within 15 minutes of TCR restimulation, both γH2AX and p-SMC-1 increased three-fold. This suggested that DNA damage and ATM activation occur in parallel, leading the authors to conclude that TCR restimulation causes DNA damage, which then triggers the ATM/p53 DNA damage response pathway.
Because it is known that AICD depends on the generation of reactive oxygen species (ROS), the investigators focused on ROS as a potential cause of DNA damage. They incubated some T cells with the antioxidant NAC prior to restimulation and compared them to T cells that were expanded using a standard protocol. Results showed that ROS levels were significantly lower in the NAC cultures than in the standard cultures. In addition, pretreatment with NAC reduced activation of the DNA damage markers, γH2AX and p-ATM, by 94% and 69%, respectively. These results confirmed that ROS generated during TCR restimulation play a central role in damaging the DNA.
This is the first study to show that expanding therapeutic T cells in the presence of NAC prior to adoptive transfer improves their ability to resist AICD. Most importantly, using these "AICD-resistant" T cells improves therapeutic outcomes in a preclinical model by enhancing T cell persistence, increasing tumor control, and improving survival.
"Now we are looking at studies to help us understand exactly how NAC changes the phenotype of T cells," said Voelkel-Johnson. "How does it make these cells survive? How is trafficking to tumors improved? There may be benefits to culturing T cells in NAC aside from generating AICD resistance that we haven't yet recognized."
More information: M. J. Scheffel et al, Efficacy of Adoptive T-cell Therapy Is Improved by Treatment with the Antioxidant N-Acetyl Cysteine, Which Limits Activation-Induced T-cell Death, Cancer Research (2016). DOI: 10.1158/0008-5472.CAN-16-0587