Discovery of molecular marker specific to early embryonic heart development
Researchers centered at Osaka University identify molecule specifically expressed on one of the first cell populations to emerge as a precursor of the heart in embryos, which enables the cells to be isolated and studied, and potentially transplanted as a treatment for heart failure.
Researchers centered at Osaka University identify a molecule specifically expressed in progenitors of the heart. This molecule, called Glial cell line-derived neurotrophic factor Family Receptor Alpha-2 (GFRA2), significantly benefits us to isolate and study pure human heart progenitors, a potentially promising source of transplant to treat heart failure in the hospitals, from pluripotent stem cells. Therefore, this discovery must push the edge of the envelope in developing a regeneration/cell replacement therapy for heart failure.
Osaka, Japan –The heart is one of the first organs to emerge as cardiac progenitors in the embryo. Thus far, various studies have increased our knowledge of how cardiac progenitors develop and then differentiate into functionally different cell types in the heart. Such knowledge is extremely critical to understand the pathological cause of congenital heart diseases as well as to produce the heart muscle cells, called cardiomyocytes, from embryonic or induced pluripotent stem (ES or iPS) cells in laboratories. Cardiomyocytes produced in laboratories are unequivocally useful for pharmacological tests without experimental animals and for the production of transplants to treat heart failure. However, our knowledge is still insufficient to apply such a treatment in the clinical arena.
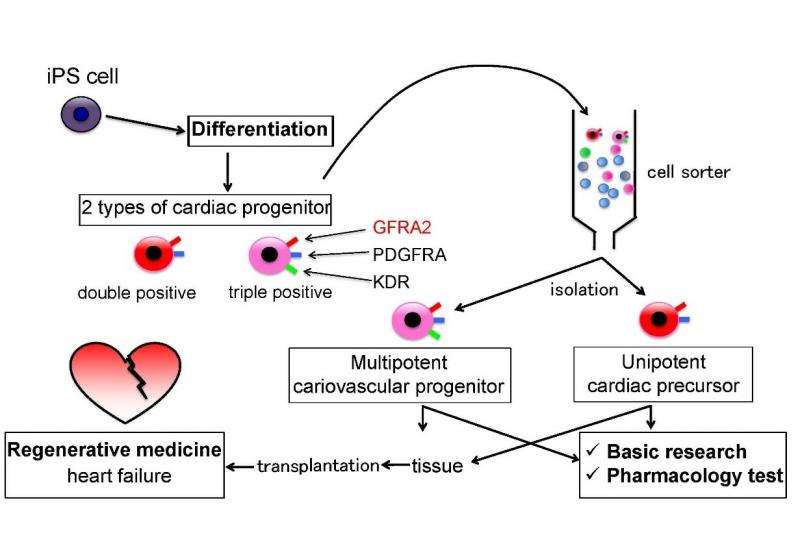
To elucidate the mechanism underlying the production and the differentiation of cardiac progenitors in detail, each of cell lineages relevant to the heart development needs to be discerned from another. If a specific "surface antigen", a molecule localized on the surface of only one lineage of interest and exposed to the outside of a cell, is available, it is extremely useful. We can easily and effectively identify, isolate, examine and/or use the cells of interest using an antibody or other agent against it. Now an international research team centered at Osaka University performed the screening to find such a useful surface antigen, and eventually found that GFRA2 specifically marks cardiac progenitors during early heart development.
The team found this protein is vital to successful heart development. Abnormal function of GFRA2 resulted in pathologically thin and less compact heart ventricle's wall. This condition, "noncompaction cardiomyopathy" causes severe heart failure with poor prognosis. Unfortunately, little is known about the pathology of this disease. Thus, elucidation of GFRA2 is expected to bring better understanding of noncompaction cardiomyopathy. More importantly, this molecule enables us to purify cardiac progenitors derived from ES and iPS cells more easily and effectively than ever, not only in mice but also in humans. Theoretically, these cardiac progenitors are promising for pharmacological test instead of experimental animals as well as for transplantation grafts of regeneration/cell replacement therapy to treat heart failure.
"We first noticed that Gfra2 was expressed specifically in cardiac progenitors but not in embryonic stem cells in both mouse and humans, and we were very excited when we knew it" corresponding author Kenta Yashiro says. "We have validated this novel finding in both mice and humans, confirmed whether an antibody for GFRA2 lets us purify cardiac progenitors of humans, and found that mice genetically engineered to lack this protein's function exhibited heart defects."
"Now we see the great potential of GFRA2. We know GFRA2 brings purer human cardiac progenitors than ever. This evidence indicates that isolated cardiac progenitors are potentially safer in terms of tumor formation because its specificity brings less chance of contamination of tumor-causing undifferentiated cells. In addition, we can purposefully harvest multipotent progenitors to produce every component of the heart or unipotent progenitors to produce only the heart muscle", Yashiro says. "Next, we will aim to completely validate the safety of cardiac progenitors isolated with GFRA2, and develop a method to use them most effectively to treat heart failure. We think our study is now about to enter an exciting stage."
More information: Hidekazu Ishida et al. GFRA2 Identifies Cardiac Progenitors and Mediates Cardiomyocyte Differentiation in a RET-Independent Signaling Pathway, Cell Reports (2016). DOI: 10.1016/j.celrep.2016.06.050