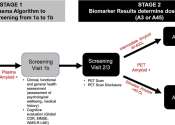
Alzheimer's drug development pipeline: Positive results, new insight on biomarkers position 2024 as 'learning year'
The world of Alzheimer's treatments is at an inflection point as more potential drugs make their way out of clinical trials.
2 hours ago
0
0