New upper limit defined for normal ALT in adolescent males
A new cohort study of adolescent offenders in Australia has identified an upper limit for ALT that is more sensitive for the diagnosis of liver disease. The findings could facilitate targeted interventions for the youths in this group, who are at high risk for HCV infection and obesity-related liver disease. The study is in the December issue of Hepatology, a journal published by John Wiley & Sons on behalf of the American Association for the Study of Liver Diseases (AASLD). The article is also available online at Wiley Interscience.
Few studies have examined the liver health of at-risk adolescents, or reported associations between liver biochemistry and hepatitis C virus or metabolic liver disease. Researchers Dr David van der Poorten and Professors Dianna Kenny and Jacob George of Sydney University, analyzed data from a large cohort of young male offenders under supervision by the New South Wales Department of Juvenile Justice in Australia, who took part in a comprehensive health study, funded by the Australian Research Council, NSW Department of Juvenile Justice and Justice Health. The researchers sought to create a new definition for the normal upper limit of liver biochemistry; to define the associations and implications of raised ALT; and to understand the risk factors and associations with hepatitis C.
They examined the results of liver tests and lipid studies from blood samples of 439 young male offenders serving community orders who agreed to participate in the health survey between October 2003 and December 2005. They also considered clinical, demographic, and lifestyle data and performed statistical analyses to determine relevant associations.
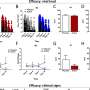
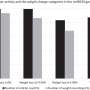
They determined the upper limit of normal for liver enzymes using the liver tests of the participants at lowest risk of liver disease. These young men had normal BMI, cholesterol, triglycerides, and blood pressure; and did not have hepatitis B or C infection, or high alcohol consumption. The researchers determined that the upper limit of normal was 28 IU/L for ALT; 32 IU/L for AST; and 29 IU/L for GGT. These are significantly lower than current upper limits for these tests which usually range from 45-55 IU/L.
Applying these cutoffs to all participants, they found that 17 percent had raised ALT, and compared to those with normal ALT, “there was a strong association for overweight or obesity,” the authors report. “To prevent further hepatic damage and to minimize cardiovascular and diabetes risk, targeted interventions in adolescents at the earliest stages of metabolic dysfunction are a particularly high priority.”
The new upper limits also detected 80 percent of the HCV-infected patients, who were significantly more likely to have injected drugs in the last 12 months. A one-year follow-up blood test of 81 of the original participants showed additional incidents of HCV antibodies, suggesting a new infection rate of at least 3.7 percent per year.
The results indicate a concerning level of HCV exposure among this population. “Hence, greater education regarding blood borne viruses, risk factors for transmission and implementing harm minimization strategies in this population is crucial,” the authors write. They also suggest routine hepatitis B vaccination.
“In conclusion,” they write, “this study has provided a wealth of clinical and health related data relevant to adolescents. The new definition of normal adolescent ALT allows greater sensitivity in diagnosing early liver disease. By identifying those with hepatitis B, C and obesity related liver disease; targeted interventions can and should be implemented to minimize future health-related morbidity.”
Related link: www.interscience.wiley.com/journal/hepatology
Source: Wiley-Blackwell