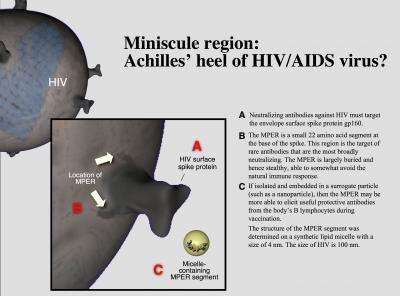
A schematic of the HIV-1 surface protein gp41 and the membrane proximal ectodomain region (MPER). Credit: Steve Moskowitz
By coaxing the HIV-1 protein to reveal a hidden portion of its protein coat, scientists at Dana-Farber Cancer Institute and Harvard Medical School have provided a newly detailed picture of how protective, or so-called broadly neutralizing, antibodies block HIV-1 infection.
In a study in the January issue of Immunity, the investigators report that the discovery may help researchers overcome two of the main stumbling blocks that have arisen on the road to an HIV vaccine: the fact that the virus's envelope protein -- the target for any antibody-based vaccine -- varies greatly from one viral strain to the next and is strewn with sugar molecules, which make it difficult for the immune system to select the virus for destruction. The paper will be posted on the journal's web site on Jan. 10 in advance of the print publication.
"Not surprisingly, only a handful of broadly neutralizing antibodies (BNAbs) have been identified, and they are rarely elicited during natural human infection," says the study’s senior author, Ellis Reinherz, MD, who is the faculty director of the Cancer Vaccine Center at Dana-Farber and a professor of medicine at Harvard Medical School.
The study focuses on an HIV-1 surface protein called gp41 and, specifically, on a portion of it known as the membrane proximal ectodomain region (MPER). This region, which lies at the base of HIV's envelope protein, is consistent across different strains of the virus. In theory, that should make it an attractive target for immune system antibodies, but, in fact, the antibody response to it is rather meager.
To determine why this is so, the Dana-Farber team studied its structure using nuclear magnetic resonance (NMR), electron paramagnetic resonance (EPR), and surface plasmon resonance (SPR) imaging techniques. They discovered that MPER is not only immersed in the viral membrane, giving it refuge from immune system attack, but it also has a hinge in the middle, which provides flexibility and helps it attach to white blood cells known as T lymphocytes.
Despite this stealthiness, the researchers found that a BNAb called 4E10 homes in on the hinge area and, in so doing, pulls out key portions of MPER that had been buried inside the membrane. Then, like a rock climber who finds an additional handhold, 4E10 latches onto these newly exposed sections, forming a tighter bond with the virus and blunting its ability to fuse with the cell membrane, the first step in viral infection.
"The new features of MPER that we've discovered may be useful targets for antibody-based vaccines if they can be held in proper configuration," says study co-author Mikyung Kim, PhD, of Dana-Farber. "One way of doing this would be to place them in a synthetic lipid coat on nanoparticles. If the antibodies aren't 'confused' by other elements of the virus's protein envelope, this approach may elicit a strong immune response to viral presence.”
Source: Dana-Farber Cancer Institute