At this week's opening of the American Association of Pharmaceutical Scientists (AAPS) Annual Meeting and Exposition (Atlanta, USA, November 16-20, 2008) Philips Research will announce its new intelligent pill technology “iPill”, targeted at assisting drug development and enabling new therapies for debilitating and life-threatening digestive tract disorders such as Crohn's disease, colitis and colon cancer.
In 2001, the first camera pill was approved by the Federal Drug Administration (FDA) for diagnostic applications. Now seven years later, for the first time, researchers from Philips will present their iPill technology – the next generation to the camera pill. The iPill is a capsule, the same size as a camera pill, and has been designed to be swallowed and to pass through the digestive track naturally. It can be electronically programmed to control the delivery of medicine according to a pre-defined drug release profile.
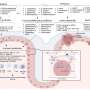
The iPill determines its location in the intestinal tract by measuring the local acidity of its environment. Distinct areas of the intestinal tract have distinct pH (a measure of acidity) profiles: the stomach is highly acidic and upon exiting the stomach the acidity of the gut sharply decreases and then becomes progressively less acidic from the upper intestine onwards. Armed with this pH information and data about capsule transit times, the location in the gut can be determined with good accuracy. The iPill releases medicine from its drug reservoir via a microprocessor controlled pump, allowing accurate programmable drug delivery. In addition, the capsule is designed to measure local temperature, and report measurements wirelessly to an external receiver unit.
“The combination of navigational feedback, electronically controlled drug delivery and monitoring of the intestinal tract promises to make iPill technology a valuable research tool for drug development,” said leading pharmaceutical drug delivery expert Dr. Karsten Cremer of Pharma Concepts GmbH, Basel (Switzerland). “In particular, I recognize the potential of this technology to improve drug candidate profiling and selection, which could ultimately accelerate the development of new drugs.”
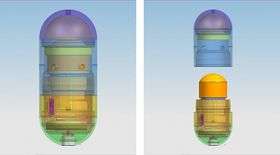
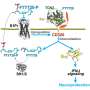
The mechanical design of Philips Research’s intelligent pill (iPill). In the form of an 11 x 26 mm capsule, the iPill incorporates a microprocessor, battery, pH sensor, temperature sensor, RF wireless transceiver, fluid pump and drug reservoir.
Philips’ intelligent pill technology
Digestive tract disorders such as Crohn's disease, colitis and colon cancer are becoming increasingly common, particularly in the western world. Crohn’s disease and colitis can be treated with drugs, notably steroids, but many of these drugs have adverse and unpleasant side effects for patients when administered systemically as whole-body doses. However, by delivering the required drugs directly to the site of disease, dose levels may be lowered and many of these side effects could be reduced.
It is this need for accurate delivery of drugs to specific sites in the intestinal tract that drove the development of Philips Research’s intelligent pill “iPill” for electronically controlled drug delivery. In addition to the potential benefits of this new technology to improve patient therapy, the iPill promises to be a valuable research tool for the development of any new drug that is delivered via the intestinal tract.
Capsules containing ultra-miniature cameras are already in use as diagnostic tools, but lack the ability to deliver drugs. The challenge for scientists at Philips Research was to find a way of navigating a drug-loaded pill capsule to the site of disease and then releasing a metered amount of drug into the gut at that location.
Navigating the gut
What Philips Research has developed is a pill that can be swallowed with food or water in the normal way and is then carried along by the normal movement of food through the gut. Knowing where the iPill is in the gut relies on the fact that the gut’s pH value (a measure of acidity) rises sharply upon exiting the stomach and becomes progressively alkaline from the upper intestine onwards. In addition, there is typically a noticeable drop in pH between the small intestine and the colon. Armed with pH information, which is measured by the iPill itself, and data about typical transit times through the gut, the iPill’s location in the gut can be determined with good accuracy. Where greater accuracy is required, external medical imaging equipment could be introduced. Locations where the drug needs to be released could also be determined by medical imaging – for example, endoscopy, MRI or CT scans.
Programmable drug release profiles
In the form of an 11 x 26 mm capsule, the iPill incorporates a microprocessor, battery, pH sensor, temperature sensor, RF wireless transceiver, fluid pump and drug reservoir. It communicates via its wireless transceiver to a control unit outside the body.
Localized drug delivery is performed by the iPill’s internal pump under the control of the microprocessor, allowing accurate control of the drug delivery profile. Examples of possible delivery profiles include a burst, progressive release or a multi-location dosing.
Pre-planning can be used to determine the target location for drug delivery and hence to define a control program for the microprocessor. This program is loaded into the iPill before it is swallowed, where it controls execution of the drug delivery profile in response to pH measurements taken as the iPill moves through the gut. Further data from the iPill, such as its temperature measurements, are reported wirelessly to an external control unit, which records data and may also transmit additional control signals back to the iPill.
Current status
Philips Research has constructed a prototype iPill capsule and system. The design of iPill is suitable for serial manufacturing. The iPill contains all the components described above and miniaturization was made possible by advanced electronic and mechanical integration. System functionality has been verified by in-vitro testing. Successful programming, measurement, and reporting functions were shown. Drug delivery was verified with model drugs using dissolution apparatus test equipment. The accuracy of the amount of drug dispensed versus time was measured and found to be better than 0.8% (average deviation over 0 - 95% volume dispensed).
Provided by Philips