Early detection of cancer: The FDA approves procedure discovered by EPFL researchers
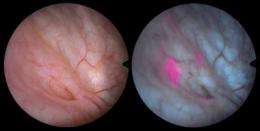
Researchers at the Ecole Polytechnique Federale de Lausanne (EPFL) in Switzerland have established a procedure where cancerous tumors in the bladder become fluorescent and are more easily discoverable under blue light. The Food and Drug Administration (FDA) approved the technique -- already in practice in Europe -- for the American market. Under the name Cysview, the product is commercialized by Photocure and GE Healthcare as the most efficient detection technique for early stage bladder cancer.
Bladder cancer is the fourth most common cancer in men and the eighth most common in women in the US. Being extremely difficult to detect in its early stages, even by the trained eye of a urologist, the importance of increasing its detectability is paramount for the complete removal of the tumors which helps to delay tumor recurrence.
Helping diagnostics and surgical removal
Cysview is instilled in the bladder. After penetrating the bladder wall it is metabolized mainly by the malignant cells, causing them to produce a fluorescent compound. The doctor then examines the bladder with an endoscope camera equipped with a blue light system: the tumors appear as easily-recognizable red spots. The procedure is not only helpful in diagnosing cancer, it is also useful for the surgeon when removing these small tumors, as he can be assured that the tumor is completely removed when no fluorescence remains.
"With Cysview, even a child can recognize these tumors", says Hubert van den Bergh, head of the project at EPFL. Then, he shows several examples where tumors have been forced out of hiding.
American experts have noticed a significant improvement with Cysview, compared to standard white light cystoscopy. A fact that is also supported in Europe with Hexvix, the European name for the product: experts have shown that the rate of recurrence of bladder cancer is significantly lower after treatment.
Application to other types of cancers
"In the future, similar procedures could be used, for instance for colon cancer or other tumors found in the hollow organs", explains Hubert van den Bergh. EPFL's Norwegian partner Photocure, owner of Hexvix/Cysview, is currently involved in developing this domain.
For the moment, this procedure is the only one of its kind approved by the FDA for use in the bladder. "There is no procedure as efficient," insists Georges Wagničres, responsible for the technology transfer in this project at EPFL, "and the market is therefore potentially very large." The approval by the FDA comes after a more than a decade of development work by the scientists at EPFL and their academic partners from the University Hospital Center (CHUV), the University of Lausanne and Photocure.














