FDA approves first human neural stem cell clinical trial to treat brain tumors
City of Hope researchers received approval from the U.S. Food and Drug Administration (FDA) to conduct the first-in-human study of a neural stem cell-based therapy targeting recurrent high-grade gliomas, the most aggressive type of brain tumor. Karen S. Aboody, M.D., associate professor in City of Hope's Department of Neurosciences, leads the research team that developed this treatment strategy. Jana Portnow, M.D., assistant professor and assistant director of the Brain Tumor Program at City of Hope, is the principal investigator for the clinical trial.
An estimated 22,500 Americans are diagnosed with malignant primary brain tumors annually, and more than 12,900 die each year from the disease. While survival rates vary with the type of brain tumor, median survival for glioblastoma, the most common type of glioma in adults, is only about 15 months. These tumors are highly invasive and ultimately resistant to current methods of treatment such as surgery, radiation and chemotherapy. One significant obstacle to curing brain tumors is the presence of the blood-brain barrier which can prevent chemotherapy agents from entering into the brain and reaching effective concentrations at tumor sites.
"This first-in-human clinical trial of a neural stem cell-based therapy that we developed at City of Hope is an investigational, targeted treatment option for recurrent high-grade glioma patients," said Aboody. "Furthermore, we envision the eventual development of neural stem cells as a platform technology for targeting multiple therapeutic agents to brain tumors, as well as other metastatic solid tumors inside and outside the brain."
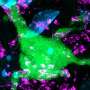
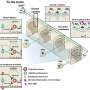
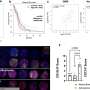
Aboody and her colleagues were the first to demonstrate in 2000 the inherent propensity of neural stem cells to home in on invasive tumor cells, also known as tropism, even migrating from the opposite side of the brain or across the blood-brain barrier. Aboody's research team has since harnessed the tumor-tropism of neural stem cells to deliver therapeutic agents to invasive tumor sites, which they demonstrated in laboratory testing. The therapy uses a genetically modified human neural stem cell line, generated by Seung U. Kim, M.D., Ph.D., professor in the Division of Neurology at the University of British Columbia, to deliver a prodrug-activating enzyme (cytosine deaminase) to brain tumor sites. This enzyme converts a relatively nontoxic prodrug (5-Fluorocytosine, 5-FC), which is delivered systemically, into an active cancer-fighting chemotherapeutic (5-Fluorouracil, 5-FU). In effect, this stem cell-mediated strategy achieves production of the chemotherapeutic drug only in the area of the tumor. This investigational treatment concentrates anticancer compounds at tumor sites while minimizing exposure of surrounding healthy tissue.
"This novel tumor-selective treatment has the potential to overcome many obstacles that limit the success of currently available treatments for malignant brain tumors and other invasive cancers," said Aboody. "Using neural stem cells as delivery vehicles for therapy may allow us to target concentrated therapeutics specifically to tumor sites while reducing the undesirable side effects of current chemotherapy regimens, including toxicity to normally dividing bone marrow, gastrointestinal tract, skin and hair cells."
The clinical trial will begin accepting patients this summer, with the goal of enrolling 12 to 20 patients with recurrent high-grade gliomas. The modified neural stem cells will be injected during surgery into the wall of the cavity remaining after tumor tissue has been removed. Study patients then receive daily doses of the prodrug 5-FC for one week. Based on Aboody's laboratory findings, once the 5-FC crosses the blood-brain barrier, the neural stem cells will convert the 5-FC to the active chemotherapy agent, 5-FU, at tumor sites in the brain. The phase I safety trial will assess the maximum tolerated dose of the therapy, and is supported by a grant from the National Cancer Institute, part of the National Institutes of Health.