Genetics underlie formation of body's back-up bypass vessels
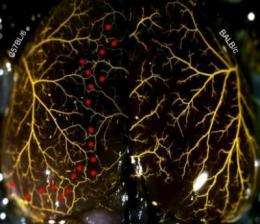
Researchers at the University of North Carolina at Chapel Hill School of Medicine have uncovered the genetic architecture controlling the growth of the collateral circulation - the "back-up" blood vessels that can provide oxygen to starved tissues in the event of a heart attack or stroke.
The new knowledge could help inform the current development of what are called collaterogenic therapies - drugs or procedures that can cause new collaterals to form and enlarge before or after a person suffers tissue damage from a blocked artery in the heart, brain, or peripheral tissues.
"This has really been the holy grail in our field, how to get new collaterals to form in a tissue with few in the first place" said senior study author James E. Faber, PhD, professor of cell and molecular physiology at UNC. "Our thesis has been that if we can figure out how these endogenous bypasses are formed in the first place in healthy tissues, what mechanisms and genetic pathways drive this, and collaterals abundance varies so widely in healthy individuals, then we may have our answer."
The results of the research, published in the August 20, 2010, issue of the journal Circulation Research, is the first to pinpoint a portion of the genome associated with variation in the density and diameter of collateral vessels.
"This may well be the seminal paper in one of the most important mysteries in vascular biology: the mechanisms controlling collateral formation in the arterial tree," wrote Stephen Schwartz, a professor of physiology at the University of Washington, in a review of the study for Faculty 1000.
The UNC research, conducted in animal models, combined classical genetic mouse crosses with a new genomic technology called association mapping to identify the section of DNA involved, starting with the whole genome, narrowing it down to several hundreds of genes and finally landing on nine candidates on mouse chromosome 7.
The researchers are now looking at these genes to see if any one of them is responsible for variation in collateral formation. Faber says they also cannot discount the possibility that it is not genes that are the deciding factor, but rather regulatory DNA or RNA elements that also reside in that same section of the genome. Either way, Faber hopes they can discover a sequence that could one day be used to predict who is most likely to develop a severe heart attack, stroke, or peripheral limb disease so those individuals can either modify their lifestyle or receive collaterogenic drugs to acquire new and potentially life-saving collateral vessels.















