Study details molecular structure of major cell signaling pathway
Scientists at the University of North Carolina at Chapel Hill School of Medicine have reported the exact molecular structure and mechanisms of a major cell signaling pathway that serves a broad range of functions in humans.
Up to half of drugs approved by the US Food and Drug Administration directly or indirectly target G protein-coupled receptors. These receptors, which are proteins that live in the outer membranes of cells, take molecular signals from outside the cell and convert them into responses within – and those responses help control behaviors as wide-ranging as cell growth, muscle contraction, platelet aggregation, sight, and smell.
Many G protein-coupled receptors engage two partners: the G protein, Gq, and an enzyme called phospholipase C, or PLC, to pass signals into the cell. Until now, it's been a mystery how this transfer of information occurs.
"We thought that to really understand how this signaling complex works, we had to go to the atomic level," said co-senior investigator Kendall Harden, PhD, Kenan Professor in the department of pharmacology at UNC.
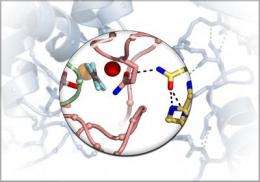
The detailed atomic structure, to be published by the journal Science, within the Science Express web site, on Thursday October 21st 2010 , "is the culmination of 15 years of work, collaboration and a small but crucial bit of educated serendipity," Harden said.
For years, the group had been trying to understand how the G protein bound to PLC. The main challenge of solving any atomic structure is to get enough of the highly purified proteins. Then, it's a matter of setting up the right conditions — a trial-and-error process of tweaking the pH of the solution, the salts and numerous other variables — to get the proteins to form a crystal that can withstand the imaging process. Robots produce thousands of slightly different chemical conditions, each produced in volumes smaller than a pinhead, and automated imaging captures each reaction.
UNC research analyst Gary Waldo, the lead author of the paper, rifled through thousands of these imaged droplets before he found one that showed the PLC bound to Gq. Enzymes had eaten away part of the PLC molecule, and this missing piece allowed the PLC to properly crystallize with its partner.
The group then created the PLC with the exact same portion missing. It bound to the G protein and formed crystals overnight, said co-investigator John Sondek, PhD, co-senior investigator and professor of pharmacology and biochemistry and biophysics at UNC. Sondek and Harden are members of the UNC Lineberger Comprehensive Cancer Center.
Once they had the structure, the researchers were able to alter parts of the complex to see exactly how they interact and how the complex works to both turn on and turn off the signal. For example, three different areas of the PLC molecule come into contact with the G protein.
To better help understand the importance of the interaction, the researchers introduced a small mutation in part of the PLC molecule they hypothesized was important to shut off the signal at the cell membrane. Expressing this mutation in the PLC of eyes of flies, the group found that the signaling pathway in eyes exposed to light stay stuck in the 'on' position. "The flies can't see because they can't refresh the signal," Sondek said.
The group plans to carry more of the molecular work into mice and other animals. "It's already well known how important the signaling pathway is, Harden said. "but our new knowledge helps us see how G proteins and PLC work together to regulate, for example, the proliferation of cells, and how certain genetic mutations in these molecules may contribute to cancer."
The new findings will also allow scientists to understand how this signaling complex interacts with other molecules. The group will eventually be able to visualize this complex interaction in real time, and to disrupt it using small compounds.
Of course, new science generates many more questions, which Harden and Sondek will pursue. "You can't be anything other than ecstatic," Harden said.















