Speed heals
USC College's Samantha Butler and collaborators show that the rate and direction of axon growth in the spinal cord can be controlled, a discovery that one day may help improve treatment for spinal injuries or neurodegenerative diseases.
Both the rate and direction of axon growth in the spinal cord can be controlled, according to new research by USC College's Samantha Butler and her collaborators.
The study, "The Bone Morphogenetic Protein Roof Plate Chemorepellent Regulates the Rate of Commissural Axonal Growth," by Butler; lead researcher Keith Phan and graduate students Virginia Hazen and Michele Frendo of USC College; and Zhengping Jia of the University of Toronto, was published online in the November 17 issue of the Journal of Neuroscience.
Butler, assistant professor of biological sciences, found that a series of connections at the cellular level produce a guidance cue that tells an axon how fast and in which direction to grow in an embryonic environment. Butler and her team also discovered that by modulating the activity of enzyme LIM domain kinase 1 (Limk1), the rate of axon growth can be stalled or accelerated.
Future applications of these findings may include enhancing the ability to regenerate neuronal circuits in patients suffering from spinal cord injuries or neurodegenerative diseases.
Initially, to understand these guidance cues, Butler and her colleagues studied the mechanisms by which neuronal circuits first develop in the embryonic states of rodents and chickens. While researching how an axon is programmed to grow in a particular direction, Butler and her group made a surprising discovery.
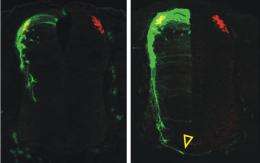
“We were expecting that when we perturbed the signaling pathway, the axon would be confused in terms of direction,” Butler said. “But we found a much greater effect — the axon grew at a different speed.”
Under normal conditions, guidance cues cause a developing neuron to extend an axon into the environment. In a developing spinal cord, the cue comes in the form of a repellant, which acts from behind the cell body to direct the growth of the axon in the opposite direction. This repellant is mediated by bone morphogenetic proteins (BMPs).
In the beginning of the multi-step growth process, BMPs bind to a cell and activate its receptors; then a second messenger is triggered, in this case Limk1. Limk1 modifies the activity of a protein called cofilin. When cofilin is active, the axon grows. If the cofilin becomes inactive, growth comes to a halt.
Butler and her team discovered that by increasing the amount of cofilin, or decreasing the amount of the restricting Limk1, the commissural axon growth accelerated. Likewise, when the amount of cofilin was decreased, or the amount of Limk1 was increased, axon growth stopped.
The axon growth in embryonic spinal cords in which Limk1 was lowered appeared to be more advanced than in controls — the axons grew up to 25 percent faster.
Since the axon is growing through an ever-changing environment, if the accelerated rate moves the axon to its subsequent signal destination too fast, that destination may not yet be created. As a result, growth acceleration can lead to errors in the process, Butler said. She hopes to determine the optimal rate of acceleration that prevents these errors but still supports enhanced regeneration.
“That the growth of axons needs to be controlled in time as well as space is something that is an interesting piece of biology,” Butler said. “How it can be applied is very exciting.”
Butler sees the application of this research as one part of the process for rebuilding damaged circuits in patients who have sustained spinal cord injuries, or those suffering from Parkinson’s or Alzheimer’s diseases, possibly using stem-cell-derived therapy. The average rate of axon growth is just 1 mm per day, so any increase would improve a patient’s treatment.
“If we knew how to modulate cofilin to maximize the speed of axon growth,” Butler said, “perhaps we could shave time off that process of circuit regeneration.”
More information: Read the full text of the article at www.jneurosci.org/cgi/content/full/30/46/15430