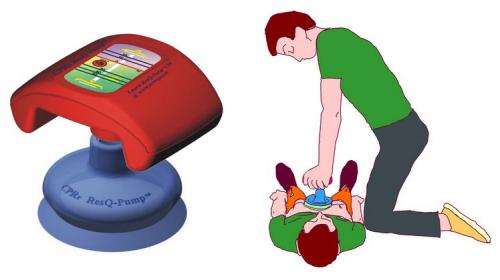
The new ResQPump device is shown in diagram (left) and in use with patient (right).
(Medical Xpress) -- When someone suffers sudden cardiac arrest outside of a hospital, their chances of survival are less than 10%, however, if being treated with two new devices instead of traditional CPR alone, they have a 53% better chance of survival. In a study published in The Lancet, Dr. Tom Aufderheide describes the devices and their results in field trials.
The ResQPump, invented after a family used a toilet plunger to administer CPR, is a hand-held device that is placed on the chest similar to where hands are placed in traditional CPR. It allows for 1 1/2 - 2" chest compressions, but rather than allowing for the chest to recoil in a passive way, the suction cup on the device provides decompression of the chest and negative intrathoracic pressure designed to return more blood to the heart.
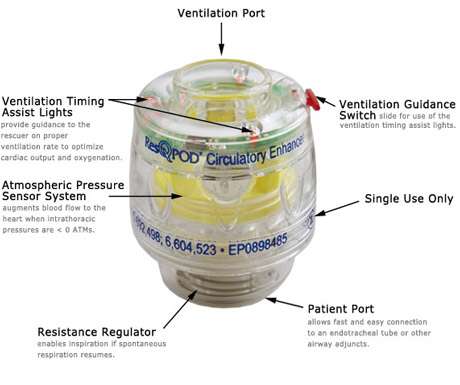
The ResQPod is used between the facemask and a ventilation bag and prevents unnecessary respiratory gases from entering the chest. It is able to create a vacuum and increase blood pressure and normal blood flow to the brain.
The ResQPod was cleared by the FDA in 2003 and the hope is the ResQPump will be FDA approved within 12-18 months.
The ResQPod
The study has included trials in numerous states including Minnesota, Washington, Michigan, Wisconsin, and Indiana. First responders in these areas rotate weeks in which they use the device combo versus standard CPR in order to provide a control group. Results have shown that 47 out of 813 in the control group survived, while the device group had 75 out of 840.
More information: Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: a randomised trial, The Lancet, Volume 377, Issue 9762, Pages 301 - 311, 22 January 2011 doi:10.1016/S0140-6736(10)62103-4 www.thelancet.com/journals/lan … (10)62103-4/fulltext
© 2010 PhysOrg.com