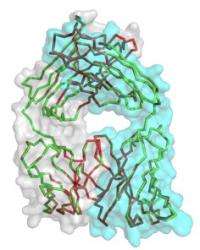
Crystal clear. A group of broadly neutralizing antibodies obtained from four unrelated slow-progressing HIV patients show striking sequence and structural similarities, suggesting that the path for the immune system to achieve this potent activity against the CD4 binding site might be narrow.
In a finding that may be good news for scientists developing HIV vaccines and therapies, a team of researchers at The Rockefeller University and the Howard Hughes Medical Institute have found a way to investigate the broadly neutralizing antibody response against the CD4 binding site of HIV on a monoclonal level. This led to the identification and characterization of several “highly active anti-CD4 binding site antibodies” (HAADs) and their expanded B cell families.
“We are excited to have the ability to isolate not only single potent antibodies but with our new approach investigate the entire families of highly active antibodies against HIV,” says first author Johannes F. Scheid. “What really surprised us was how similar these antibodies were even though they came from different donors, so the path to make them seems to be quite narrow.”
The new research builds on previous findings from the Laboratory of Molecular Immunology, headed by Sherman Fairchild Professor Michel C. Nussenzweig. Scheid and colleagues first characterized in 2009 the overall B cell repertoire against the HIV surface protein in patients with broadly neutralizing antibodies on a monoclonal level. These patients are a group of individuals that make up roughly 10 percent of HIV patients and their antibodies are able to kill a wide range of diverse HIV strains. Later, Hugo Mouquet and colleagues in Nussenzweig’s lab investigated how polyreactivity might be selected for in the antibody response against HIV. In related research, John Pietzsch and colleagues described the fine epitope of one group of HIV neutralizing antibodies.
For the latest research, Nussenzweig and Scheid, along with David D. Ho of Rockefeller and the Aaron Diamond AIDS Research Center and Rockefeller’s Brian T. Chait, looked at the immune system of four unrelated slow-progressing HIV patients. The patients produced expanded clones of potent broadly neutralizing CD4 binding site antibodies that mimic binding to CD4, the receptor that HIV uses to gain entry into host T-cells. The researchers cloned 576 new HIV antibodies, which were derived from a small number of germ-line immunoglobulin genes. In addition, despite undergoing extensive hypermutation, these antibodies show striking sequence and structural similarities suggesting that the path for the immune system to achieve this potent activity against the CD4 binding site might be narrow.
The researchers also found that relatively low concentrations of these broadly neutralizing antibodies are required to neutralize the virus.
“This work will help us to understand better how broad neutralization is achieved in some patients and how to possibly phenocopy such a response by a vaccine strategy,” says Scheid.
More information: Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding, Johannes F. Scheid, et al. Science online: July 14, 2011
Provided by Rockefeller University