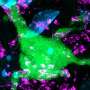
A study by researchers at the University of California, San Diego School of Medicine may lead to a way to prevent the progression, or induce the regression, of lung injury that results from use of the anti-cancer chemotherapy drug Bleomycin. Pulmonary fibrosis caused by this drug, as well as Idiopathic Pulmonary Fibrosis (IPF) from unknown causes, affect nearly five million people worldwide. No therapy is known to improve the health or survival of patients.
Their research shows that the RSK-C/EBP-Beta phosphorylation pathway may contribute to the development of lung injury and fibrosis, and that blocking this phosphorylation -- a regulatory mechanism in which proteins and receptors are switched on or off -- improved Bleomycin-induced lung fibrosis in mice. The study appears on-line October 5 in Proceedings of the Library of Science (PloS ONE).
Bleomycin is a common chemotherapy drug used to treat many forms of cancer, according to study authors Martina Buck, PhD, associate professor of medicine, and Mario Chojkier, MD, professor of medicine, both researchers at UC San Diego Moores Cancer Center and the VA San Diego Healthcare System. "Unfortunately, use of Bleomycin has damaging side effects, including lung fibrosis. We are hopeful that this discovery could provide a way to stop such lung damage so that cancer patients could better tolerate this chemotherapy," said Buck.
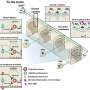
The downstream molecular mechanism that causes Bleomycin-induced lung fibrosis remained unknown. The scientists set out to identify the specific signaling involving a single amino acid within a specific domain of one protein that could be blocked the half the progression of such injury, in order to design effective targeted therapeutics.
They found that blocking RSK phosphorylation of a binding protein called C/EBP-Beta on the RSK macromolecule Thr217 with either a single point mutation or a blocking peptide ameliorated the progression of lung injury and fibrosis induced by Bleomycin in mice.
"We hypothesized that this pathway was critical given similarities between liver and lung fibrogenesis. RSK plays an important role in both the macrophage inflammatory function and survival of activated liver myofibroblasts -- cells that contribute to maintenance and tissue metabolism," said Buck. "Therefore, we proposed that a similar signaling mechanism is also responsible for lung injury and fibrosis."
By identifying the peptide that shuts down this process, the researchers were essentially able to sequester a small piece of an important regulatory protein, C/EBP Beta, responsible for fibrosis, thereby stopping phosphorylation. "Basically, the kinase protein gets hung up, trying again and again -- unsuccessfully -- to 'turn on' the fibrous growth," Buck added.
In addition, phosphorylation of human C/EBP-Beta was induced in human lung fibroblasts in culture and in situ in lungs of patients with severe lung fibrosis, but not in control lungs, suggesting that this signaling pathway may be also relevant in human lung injury and fibrosis.
The researchers add that it is premature to assess whether this pathway will provide an effective therapeutic target. However, blocking progression of lung fibrosis could decrease the need for lung transplantation, since IPF is the main indication for lung transplants worldwide.
Provided by University of California - San Diego