Whitehead Institute scientists have determined that master transcription factors determine the genes regulated by key signaling pathways. In this way, signaling pathways are targeted to genes that are most relevant to each cell type and tailor gene expression to control cell state, growth, differentiation, and death.
Normal development, from fertilized egg to adult organism, depends on each cell receiving proper instructions from its environment. In response to such incoming information, receptors on a cell's surface send signals to the nucleus that tweak gene expression and control cellular function.
However, in a number of human diseases, including cancer, cell signaling pathways can go awry. Without the correct information making its way into the nucleus, gene expression is altered, often with dire consequences.
Although researchers have long understood the importance of these signaling pathways, the mechanism through which they actually affect gene expression had been unclear. In research published this week in the journal Cell, scientists in the lab of Whitehead Institute Member Richard Young describe how a protein acts as a courier, carrying a message from a receptor on the cell's surface to a master transcription factor on the cell's DNA. The courier then tailors expression of genes bound by master transcription factors.
The discovery sheds new light on the relationship between signaling pathways, gene expression, cell function, and disease—at the same time revealing potential targets for therapeutic intervention and novel approaches for reprogramming neurons or insulin-producing beta cells to treat nerve damage or diabetes.
"This is a broad, simplifying concept that is key to understanding how the whole human system works and how the genome responds to the world around it—that each signaling pathway has its own signaling molecule that finds its way to that cell's type-specific master transcription factor," says Young, who is also a professor of biology at MIT. "This idea allows us to think clearly about what is going awry in disease and how we potentially can treat people by modifying these signaling pathways."
To tease apart how signaling pathways modulate gene expression, Alan Mullen, who is the Cell paper's first author and a visiting scientist in the Young lab, analyzed the transforming growth factor-beta (TGF-beta) signaling pathway in several types of mouse and human cells. In both developing and mature cells, the TGF-beta pathway is involved in numerous cell activities, including specialization, homeostasis, and programmed death. Yet, corrupted versions of the TGF-beta signaling pathway are frequently found in cancer cells, allowing the cancer cells to proliferate and escape normal programmed cell death.
Mullen knew that when the TGF-beta signaling pathway's receptor is triggered by an environmental change, it activates the SMAD3 protein, which somehow couriers the receptor's message to the cell's DNA. Previous research had also suggested that SMAD3 might interact with hundreds of different transcription factors to achieve the desired response to environmental input.
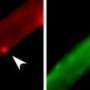
Looking at embryonic stem, muscle, and certain immune system cells, Mullen found that once inside the nucleus, the activated SMAD3 settles onto the DNA adjacent to a master transcription factor, the main switch that turns the genes specific to each cell type on or off. In embryonic stem cells, SMAD3 nestles next to the master transcription factor Oct4.
"We were really surprised when we looked at the data because SMAD3 wasn't distributed with many different transcription factors. Instead, it appeared to be following Oct4, which is one of the transcription factors that determines embryonic stem cell state," says Mullen. "The master transcription factors are expressed at very high levels and dominate the transcription machinery because there is so much of these factors."
In muscle cells and specific immune system cells, Mullen saw similar results. SMAD3 only bound to the DNA next to the master transcription factors Myod1 and PU.1, respectively. Once associated with SMAD3, the master transcription factors adjust the nearby genes' expression.
Mullen's work indicates that a signal from one signaling pathway can interact with different master transcription factors in different cell types, which explains why the same signal can have distinct effects in multiple cell types. And the signal interacts with just one or a few master transcription factors in each cell type—not hundreds of transcription factors.
Related research from Young postdoctoral researcher Lee Lawton and graduate student Zi Peng Fan, who collaborated with the lab of Leonard Zon at Children's Hospital Boston, supports Mullen's findings. In an article in the same issue of Cell, the team's work with maturing blood cells shows that the BMP and Wnt signaling pathways use their respective signal molecules throughout the blood maturation process, but that the signals target various master transcription factors based on the cells' stage in this process.
More information: "Master Transcription Factors Determine Cell-Type-Specific Responses to TGF-β Signaling" Cell, October 28, 2011.
Provided by Whitehead Institute for Biomedical Research