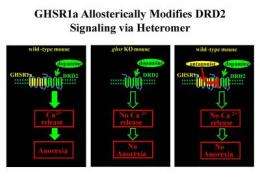
GHSR1a allosterically modifies DRD2 signaling via a heteromer. Credit: Roy G. Smith, The Scripps Research Institute
New research reveals a fascinating and unexpected molecular partnership within the brain neurons that regulate appetite. The study, published by Cell Press in the January 26 issue of the journal Neuron, resolves a paradox regarding a receptor without its hormone and may lead to more specific therapeutic interventions for obesity and disorders of dopamine signaling.
Ghrelin is an appetite-stimulating hormone produced by the stomach. Although the ghrelin receptor (GHSR1a) is broadly distributed in the brain, ghrelin itself is nearly undetectable there. This intriguing paradox was investigated by Dr. Roy G. Smith, Dr. Andras Kern, and colleagues from The Scripps Research Institute in Florida. "We identified subsets of neurons in the brain that express both GHSR1a and the dopamine receptor subtype-2 (DRD2)," explains Dr. Smith. "Dopamine signaling in the hypothalamus is linked with feeding behavior, and mutations in DRD2 that attenuate dopamine signaling are associated with obesity in humans. We speculated that expression of both receptors in the same neurons might lead to interactions between GHSR1a and DRD2 that modify dopamine signaling."
The researchers showed that when GHSR1a and DRD2 were coexpressed, the receptors physically interacted with one another. Further, the GHSR1a:DRD2 complex was present in native hypothalamic neurons that regulate appetite. When mice were treated with a molecule (cabergoline) that selectively activates DRD2, they exhibited anorexia. Interestingly, the cabergoline-stimulated anorexia did not require ghrelin but was dependent on GHSR1a and the GHSR1a:DRD2 interaction. These findings suggest that in neurons expressing both GHSR1a and DRD2, GHSR1a alters classical DRD2 dopamine signaling.
"Perhaps most importantly, we showed that a GHSR1a-selective antagonist blocks dopamine signaling in neurons with both DRD2 and GHSR1a, which allows neuronal selective fine-tuning of dopamine signaling because neurons expressing DRD2 alone will be unaffected," concludes Dr. Smith. "This provides exciting opportunities for designing next-generation therapeutics with fewer side effects for both obesity and psychiatric disorders associated with abnormal dopamine signaling."
More information: Kern et al.: “Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism.” Neuron, January 26, 2012.
Journal information: Neuron
Provided by Cell Press