Exercise triggers beneficial cellular recycling: study
Everyone knows exercise is good for you. We’re told time spent on the treadmill can reduce our risk of diabetes, cancer, and cardiovascular disorders. But exactly how exercise provides this protection is a bit of a mystery. A new study finds that exercise prompts cells to break down unwanted proteins and other cellular junk to produce more energy. The process, called autophagy, may explain how exercise fends off metabolic disorders like diabetes and protects against other diseases.
Autophagy is like a “cellular garbage disposal,” says Howard Hughes Medical Institute investigator Beth Levine, a physician at the University of Texas Southwestern Medical School in Dallas who has been studying the process for more than a decade. The process works like this: First, a double membrane forms around the unwanted cargo inside the cell, enveloping it. This membrane then fuses with an organelle called a lysosome, which contains enzymes that rush in and break down the contents. The bits and pieces created by this process get recycled, providing raw materials for new structures or a burst of energy.
Autophagy keeps cells healthy by “getting rid of all of the obsolete and abnormal structures,” Levine explains. It also helps cells survive lean times. By cannibalizing unwanted proteins and other junk, the cells can get nutrients.
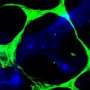
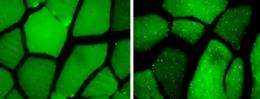
Scientists have long known that starvation can trigger a boost in autophagy. Levine and her colleagues suspected that exercise, because it increases cells’ energy demands, might have a similar effect. To test the idea, she and her colleagues used transgenic mice whose cells produce a glowing green protein whenever autophagy occurs. Then they placed these mice on treadmills. After 30 minutes of running, the rodents’ muscle and heart cells were speckled with green dots, evidence of increased autophagy. “That was a brand new finding,” she says. In a paper published January 18, 2012, in the journal Nature, the researchers report that exercise also sparked an increase in autophagy in cells in the liver and pancreas, organs involved in the metabolism of glucose.
Next, Levine and her colleagues set out to determine what purpose the autophagy serves. The team engineered mice that could undergo normal autophagy, but due to a mutation in a gene called B-cell lymphoma 2 (Bcl-2), lacked the ability to ramp up autophagy during exercise or starvation. Bcl-2 is known to inhibit cell death and plays a key role in regulating autophagy.
When the researchers placed these mutant mice on the treadmill, they found that they couldn’t run as long as normal mice. A closer look revealed that the mice weren’t metabolizing sugar properly. When a normal mouse runs on a treadmill, its muscle cells kick into overdrive, increasing their uptake of sugar. Consequently, the amount of sugar in the blood plummets. But this didn’t happen in the mutant mice. The enzyme that helps cells take in more sugar, called AMP kinase, did not get activated. As a result, the blood sugar levels of the mice stayed unnaturally high and the mice showed less endurance.
Levine thought that these mutant mice might not derive any long-term benefit from exercise either. To test the hypothesis, the researchers fed both mutant and normal mice a high-fat diet for four weeks. Not surprisingly, the mice gained weight and developed a disorder akin to type 2 diabetes, a disease in which sugar doesn’t move efficiently from the blood into cells.
Next, they put the mice on a stringent exercise regimen for eight weeks while still feeding them a high fat diet. The normal mice lost weight and their diabetes disappeared. Their muscle cells regained the ability to take up sugar. The mutant mice that exercised on the treadmill also lost weight, but they didn’t get any of the metabolic benefits; their blood sugar levels stayed high. The data suggest that, to get the benefits, “autophagy really was necessary,” Levine says.
The findings suggest that increased autophagy may be the reason why exercise protects against type 2 diabetes and other metabolic disorders. Several oral drugs used to treat type 2 diabetes work by activating AMP kinase. Autophagy induced by exercise appears to do the same thing.
The team’s next step will be to investigate whether autophagy could also explain why regular exercise protects against cancer, neurodegenerative disorders, and aging. For cancer, the mechanism may be similar, Levine speculates. Studies have shown that diabetics who take AMP kinase activators have a lower incidence of cancer than diabetics with similar blood sugar levels who don’t take the drugs.
In the meantime, Levine plans to get more exercise herself. She recently invested in a treadmill. “If it’s good enough for my mice,” she says, “it’s good enough for me.”
More information: Study: DOI:10.1038/nature10758