Parkinson's protein causes disease spread in animal model, suggesting way disorder progresses over time in humans
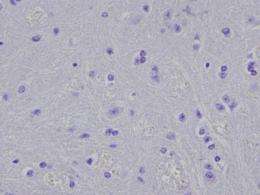
(Medical Xpress) -- Penn researchers have shown that brain tissue from a Parkinson's disease mouse model , as well as synthetically produced disease protein fibrils, injected into young, symptom-free PD mice led to spreading of PD pathology.
Last year, researchers from the Perelman School of Medicine at the University of Pennsylvania found that small amounts of a misfolded brain protein can be taken up by healthy neurons, replicating within them to cause neurodegeneration. The protein, alpha-synuclein (a-syn), is commonly found in the brain, but forms characteristic clumps called Lewy bodies, in neurons of patients with Parkinson's disease (PD) and other neurodegenerative disorders. They found that abnormal forms of a-syn called fibrils acted as "seeds" that induced normal a-syn to misfold and form aggregates.
In earlier studies at other institutions, when fetal nerve cells were transplanted into the brains of PD patients, some of the transplanted cells developed Lewy bodies. This suggested that the corrupted form of a-syn could somehow be transmitted from diseased neurons to healthy ones.
Now, in a follow-up study published in the Journal of Experimental Medicine, the team, led by senior author Virginia M.-Y Lee, PhD, director of the Center for Neurodegenerative Disease Research and professor of Pathology and Laboratory Medicine, showed that brain tissue from a PD mouse model , as well as synthetically produced a-syn fibrils, injected into young, symptom-free PD mice led to spreading of a-syn pathology. By three months after a single injection, neurons containing abnormal a-syn clumps were detected throughout the mouse brains. The inoculated mice died between 100 to 125 days post-inoculation, out of their typical two-year life span.
"We think the spreading is via white-matter tracks through brain neural network connections," explains Lee. "This study will open new opportunities for novel Parkinson's disease therapies."
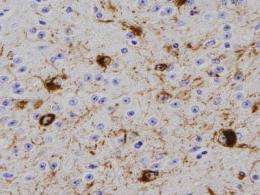
One of the remaining questions is how, once inside a neuron, does the misfolded a-syn protein spread from cell to cell.
"It's like a biochemical chain reaction," says first author Kelvin C. Luk, Ph.D., research associate, in the CNDR. Once inside the confines of a neuron, the misfolded a-syn recruits normally shaped a-syn protein that is present in the cell, causing them to eventually misfold. This occurs along the axons and dendrites (neuronal extensions that reach other neurons), leading to a dramatic accumulation of the abnormal protein. The misshapen a-syn then invades other neurons when they reach the synapse, the small space between neurons.
This transmission process is remarkably similar to what is seen in prions, the protein agents responsible for conditions such as transmissible spongiform encephalopathies ( mad cow disease). However, the researchers are quick to caution that there is no evidence that Parkinson's or any related neurodegenerative diseases is either infectious or acquired.
The accumulation of misfolded proteins is a fundamental pathogenic process in neurodegenerative diseases, but the factors that trigger aggregation of a-syn are poorly understood.
The Penn team saw that misfolded a-syn propagated along major central nervous system pathways, reaching regions far beyond injection sites. What's more, they showed for the first time that synthetically produced a-syn fibrils are sufficient to initiate a vicious cycle of Lewy body formation and transmission of the misfolded a-syn in mice.
The study demonstrates just how the Parkinson's disease protein can spread in a patient's brain in terms of uptake into a healthy neuron, expansion within the cell, and finally release to a neighboring neuron.
"Knowing this mechanism allows for possible immunotherapies to interrupt the chain reaction by stopping the mutant protein from spreading at the synapse," says Lee.
"Shedding light on how α-synuclein contributes to Parkinson's disease and related Lewy body disorders is of significant interest both for understanding these diseases and developing potential treatments," said Beth-Anne Sieber, Ph.D., of the National Institute of Neurological Disorders and Stroke (NINDS), part of the National Institutes of Health. "This study provides evidence for the progressive, pathological spread of α-synuclein through the brain."















