How aging normal cells fuel tumor growth and metastasis
It has long been known that cancer is a disease of aging, but a molecular link between the two has remained elusive.
Now, researchers at the Kimmel Cancer Center at Jefferson (KCC) have shown that senescence (aging cells which lose their ability to divide) and autophagy (self-eating or self-cannibalism) in the surrounding normal cells of a tumor are essentially two sides of the same coin, acting as "food" to fuel cancer cell growth and metastasis.
Michael P. Lisanti, M.D., Ph.D., Professor and Chair of Stem Cell Biology and Regenerative Medicine at Jefferson Medical College of Thomas Jefferson University and a member of the KCC, and his team previously discovered that cancer cells induce an oxidative stress response (autophagy) in nearby cells of the tumor microenvironment to feed themselves and grow.
In this study, senescent cells appear to have many of the characteristics of these autophagic cancer-associated fibroblasts and to be part of the same physiological process. In other words, normal neighboring cells that are becoming senescent or "old" are directly making food to "feed" the cancer. Aging literally fuels cancer cell growth.
Since senescence is thought to reflect biological aging, this research on autophagy-induced senescence may explain why cancer incidence dramatically increases exponentially with advanced age, by providing a "fertile soil" to support the anabolic growth of "needy" cancer cells.
The findings were reported in the June 15 issue of Cell Cycle.
"This research merges the two paradigms of aging and cancer, and it also brings in cell metabolism," said Dr. Lisanti. "We provide genetic support for the importance of 'two-compartment tumor metabolism' in driving tumor growth and metastasis via a very simple energy transfer mechanism. Senescence and autophagy metabolically support tumor growth and metastasis."
Simply put, aging is the metabolic engine that drives cancer growth.
To test this link, the researchers developed a genetically tractable model system to directly study the compartment-specific role of autophagy in tumor growth and metastasis. First, they took human fibroblasts immortalized with telomerase and transfected them with autophagy genes.
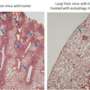
Next, they validated that these fibroblasts show features of mitophagy, mitochondrial dysfunction and a shift toward aerobic glycolysis, with increases in lactate and ketone production, mimicking the behavior of cancer-associated fibroblasts. They observed that autophagic-senescent fibroblasts promoted metastasis, when co-injected with human breast cancer cells, by more than 10-fold.
Thus, metastasis may be ultimately determined by aging or senescent cells in the tumor microenvironment, rather than by the cancer cells themselves. This finding completely changes how we view cancer as a disease.
This observation directly calls into question the longstanding notion that cancer is a cell-autonomous genetic disease. Rather, it appears that cancer is really a disease of host aging, which fuels tumor growth and metastasis, thus, determining clinical outcome. Normal aging host cells are actually the key to unlocking effective anti-cancer therapy.
In the study, the autophagic fibroblasts also showed features of senescence. What's more, the senescent cells shifted toward aerobic glycolysis, and were primarily confined to the tumor stromal compartment.
Autophagy action is also clearly compartment specific, since the researchers showed that autophagy induction in human breast cancer cells resulted in diminished tumor growth. Therefore, selective induction of self-cannibalism in cancer cells is a new therapeutic target for the prevention of tumor growth and metastasis. In this strategy, cancer cells actually eat themselves, reversing tumor growth and metastasis.
To stop tumor growth and metastasis, researchers will need to "cut off the fuel supply" which is provided by aging senescent cells, before it gets to cancer cells by targeting autophagy and senescence in the tumor microenvironment.
These findings are paradigm shifting and will usher in a completely new era for anti-cancer drug development, according to the researchers. Such approaches for targeting the "autophagy-senescence transition" could have important implications for preventing tumor growth and metastasis, and effectively overcoming drug resistance in cancer cells.
"Rapidly proliferating cancer cells are energetically dependent on the aging host tumor stroma," Dr. Lisanti said. "As such, removing or targeting the aging tumor stroma would then stop tumor growth and metastasis. Thus, the aging stroma is a new attractive metabolic or therapeutic target for cancer prevention."
A clear byproduct of this research would also be the development new anti-aging drugs to effectively combat, stop or reverse aging, thereby preventing a host of human diseases, particularly cancer.
More information:
Key References: Capparelli et al.
www.landesbioscience.com/journ … ls/cc/article/20717/
www.landesbioscience.com/journ … ls/cc/article/20718/
www.landesbioscience.com/journ … ls/cc/article/20920/
www.landesbioscience.com/journ … ls/cc/article/20964/