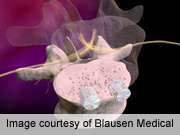
A two-dimensional fluoroscopic technique is safe for percutaneous placement of pedicle screws during transforaminal lumbar interbody fusion, with a pedicle breach rate of 6.2 percent, according to a study published online June 7 in the Journal of Spinal Disorders & Techniques.
(HealthDay) -- A two-dimensional (2D) fluoroscopic technique is safe for percutaneous placement of pedicle screws during transforaminal lumbar interbody fusion (TLIF), with a pedicle breach rate of 6.2 percent, according to a study published online June 7 in the Journal of Spinal Disorders & Techniques.
Zachary A. Smith, M.D., from Northwestern University in Chicago, and colleagues conducted a prospective study involving 151 patients (average age, 56.6 years) following instrumented single-level (129 cases) or two-level (22 cases) minimally-invasive TLIF. A total of 601 pedicle screws were used for percutaneous fixation, with placement guided by 2D fluoroscopy. Patients were followed for potential clinical symptoms, and radiographic results, including postoperative computed tomography scans, were analyzed.
The researchers observed 37 pedicle breaches in 601 instrumented pedicles (6.2 percent), with 22 significant breaches (≥3mm; 3.7 percent). The level of the breaches were L3 (five of 46; 10.2 percent), L4 (12 of 201; 7.0 percent), L5 (15 of 158; 9.5 percent), and S1 (three of 47; 3.4 percent). There were 22 medial breaches, 12 lateral, two superior, and one inferior. Two of the breaches were symptomatic; both were associated with a medial breach at the L5 pedicle. Symptoms were transient and neither breach required hardware repositioning.
"In the current study, we report a pedicle breach rate of 6.2 percent using a 2D fluoroscopic technique without computer assistance," the authors write. "While newer assistive technologies, including computer assisted fluoroscopy and the O-arm, may decrease occupational exposure, the accurate placement of pedicle screws can be undertaken without this technology."
One author disclosed financial ties to Medtronic.
More information:
Abstract
Full Text (subscription or payment may be required)
Copyright © 2012 HealthDay. All rights reserved.