Failing metal hip implants could be releasing genotoxic material
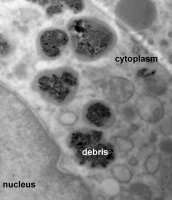
(Medical Xpress) -- Scientists have discovered that the inflammation in the surrounding tissue of patients with failing chromium-cobalt metal-on-metal (MOM) implants is caused by the release of The Cobalt 2+ ions (Co2+) from metal debris that wears away from replacement joints. These ions are known to be genotoxic and could lead to further medical complications.
Approximately 10,000 patients in the UK have had cobalt-chromium alloy MOM hip implants, which were recalled in 2010 because rubbing between the components caused nanoscopic metal debris to be released in surrounding tissue, causing chronic inflammation and loss of mobility in patients. To date, 7,500 patients in the UK have had MOM implants removed and replaced with newer implants. However, the mechanism by which the inflammation is caused has not been fully understood.
Now, researchers from Imperial College London and Ohio State University have used a new approach that combines high resolution X-ray and electron microscopy to determine the cause of the chronic inflammation in tissue samples from affected patients. They discovered that residual chromium is oxidised and Co2+ions are released as the nanoparticles corrodes in the tissue, which is the cause of the inflammation. Previous studies have shown that Co2+ions are genotoxic, which could potentially damage DNA and lead to further long-term medical complications in patients.
The study, published online this month in the journal Chemical Communications, is one of the first to look at the effects of nano-particles in humans and raises questions about how materials are tested before they are used as implantable materials.
Dr Mary Ryan, co-author of the paper from the Department of Materials at Imperial College London, says:
“We were able to meet patients who had these failing implants and we could see first-hand the chronic inflammation, pain and loss of mobility they experienced. Even though a huge number of patients have benefited from replacement surgery, we still don’t fully understand the long-term impacts that implantable materials have on our bodies. Our work is one of the first to study these nanoparticles and the effects that they have on damaged cells and tissue. This has enabled us to understand in much more detail the side effects that these materials may have in patients.”
Dr Alexandra Porter, co-author also from the Department of Materials at Imperial, adds: “There is a double edged sword to these findings because on the one hand, we’ve found a root cause of inflammation, which may lead to better intervention therapies for patients. On the other, although we still need to do more work to understand the full impact; our results suggest that these nano-particles may have a long-term genotoxic impact on patients.”
The researchers in today’s study found the nano-particles had accumulated in white blood cells, whose job it is to clean up debris in the body. Here they undergo a corrosion process where the cobalt dissolves rapidly and is released into the surrounding tissue and blood stream. The less soluble chromium forms a solid residue that remains in the tissue.
The team needed to understand the chemical make-up of the nanoparticles in the tissue as well as structural information about their size and distribution. They took tissue samples to the Canadian Light Source - Canada’s national synchrotron research facility and one of the few facilities in the world where materials can be studied at a high enough resolution to see the nano-particles. They used the high resolution X-ray microscopes to scan the tissue to determine the chemistry of the nano-particles. The researchers then analysed the same samples in the Titan microscope at Imperial in the UK. This two-step process, which hasn’t been used before, enabled the researchers to build a highly detailed picture of how the nano-particles corroded in the tissue samples.
The next step will see the team carrying out further research to understand why these nanoparticles are corroding while the bulk of the alloy in MOM hip implants is corrosion resistant. The researchers also aim to use this correlative approach to explore other diseases where nanoscale materials may have an impact on human health, such as Alzheimer’s disease.
In the long-term, the researchers aim to combine their technique with Magnetic Resonance Imaging, which could be used predict and detect long-term damage in patients with hip replacements to improve clinical outcomes.

















