deCODE Genetics, together with their colleagues from the pharmaceutical company Genentech, reported today in the journal Nature the discovery of a variant of the amyloid precursor protein (APP) gene that confers protection against both Alzheimer's disease (AD) and cognitive decline in the elderly. The findings also indicate a linkage between age-related cognitive decline and late-onset forms of AD, the most common cause of dementia.
"Our results suggest that late-onset Alzheimer's disease may represent the extreme of more general age-related decline in cognitive function," said study lead author Kari Stefansson, M.D., Dr. Med., CEO of deCODE Genetics. "Also important, these data support certain Alzheimer's disease drug development programs--some of which are already in human clinical trials."
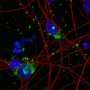
Alzheimer's disease is a progressive neurodegenerative disease associated with the production and accumulation of beta-amyloid peptides produced by cleaving bits off the APP. While several mutant forms of the APP gene have been linked to early-onset, aggressive forms of AD, there is limited evidence supporting a role for mutations in the gene in the more common late-onset form of the disease.
In searching for low-frequency variants of the APP gene associated with AD, deCODE scientists found a significant association with a mutation in whole genome sequence data from 1,795 Icelanders. The research team showed that the mutation is significantly more common in the study's elderly control group than in those with AD, suggesting that the mutation confers protection against the disease.
The Genentech team then tested these findings using in vitro cellular assays with wild-type APP and APP enriched with A673T, the mutation allele. Importantly, they showed a significantly reduced production of amyloid beta in cells with A673T.
"Our genetic data indicate that the mutation is protective against Alzheimer's disease," said Stefansson. "Our findings and the in vitro work done by Genentech also provide a proof of principle for the idea that blocking BACE1 cleavage of APP may protect against Alzheimer's, offering greater confidence to pharmaceutical companies with active BACE1 inhibitor drug development programs."
To study the association of the protective mutation with general cognitive decline, the research team examined the frequency of the mutation in the original Icelandic control group of those cognitively intact at age 85. The team found an enrichment of the mutation in this group, consistent with its protective effect against AD.
Extending this work further, the team investigated cognitive function using a seven-category test in carriers of the mutation and non-carriers in the age range of 80 to 100 years old. The research team found a statistically significant difference between carriers and non-carriers, with the carriers of the mutation having a score indicative of better-conserved cognition. After removing known AD cases, the team again found that carriers had better cognitive function, suggesting that the mutation extends its protective effect to the elderly in general.
"The implication of these data is that general cognitive decline and late-onset Alzheimer's disease share biological pathways," said Stefansson. "It also suggests that approaches to treating Alzheimer's may have benefit to those elderly who do not carry the protective mutation, and do not suffer from AD."
Alzheimer's disease, a progressive neurodegenerative disorder, is the most common form of dementia that affects four to eight percent of the elderly population worldwide. The neuropathological features of AD are the presence of extracellular amyloid plaques and intracellular neurofibrillary tangles in the hippocampus and cortical grey matter of the AD brain.
Age-specific prevalence of AD nearly doubles after age 65, leading to a prevalence of greater than 25 percent in those over the age of 90.
More information: DOI: 10.1038/nature11283
Journal information: Nature
Provided by deCODE Genetics