A new study raises concern about chronic exposure of workers in industry to a food flavoring ingredient used to produce the distinctive buttery flavor and aroma of microwave popcorn, margarines, snack foods, candy, baked goods, pet foods and other products. It found evidence that the ingredient, diacetyl (DA), intensifies the damaging effects of an abnormal brain protein linked to Alzheimer's disease. The study appears in ACS' journal Chemical Research in Toxicology.
Robert Vince and colleagues Swati More and Ashish Vartak explain that DA has been the focus of much research recently because it is linked to respiratory and other problems in workers at microwave popcorn and food-flavoring factories. DA gives microwave popcorn its distinctive buttery taste and aroma. DA also forms naturally in fermented beverages such as beer, and gives some chardonnay wines a buttery taste. Vince's team realized that DA has an architecture similar to a substance that makes beta-amyloid proteins clump together in the brain — clumping being a hallmark of Alzheimer's disease. So they tested whether DA also could clump those proteins.
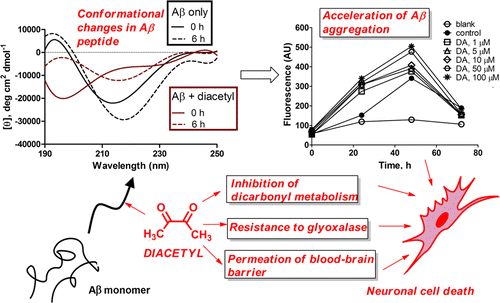
DA did increase the level of beta-amyloid clumping. At real-world occupational exposure levels, DA also enhanced beta-amyloid's toxic effects on nerve cells growing in the laboratory. Other lab experiments showed that DA easily penetrated the so-called "blood-brain barrier," which keeps many harmful substances from entering the brain. DA also stopped a protective protein called glyoxalase I from safeguarding nerve cells. "In light of the chronic exposure of industry workers to DA, this study raises the troubling possibility of long-term neurological toxicity mediated by DA," say the researchers.
More information: “The Butter Flavorant, Diacetyl, Exacerbates ß-Amyloid Cytotoxicity” Chem. Res. Toxicol., Article ASAP. DOI: 10.1021/tx3001016
Abstract
Diacetyl (DA), an ubiquitous butter-flavoring agent, was found to influence several aspects of amyloid-β (Aβ) aggregation—one of the two primary pathologies associated with Alzheimer's disease. Thioflavin T fluorescence and circular dichroism spectroscopic measurements revealed that DA accelerates Aβ1–42 aggregation into soluble and ultimately insoluble β-pleated sheet structures. DA was found to covalently bind to Arg5 of Aβ1–42 through proteolytic digestion–mass spectrometric experiments. These biophysical and chemical effects translated into the potentiation of Aβ1–42 cytotoxicity by DA toward SH-SY5Y cells in culture. DA easily traversed through a MDR1-MDCK cell monolayer, an in vitro model of the blood–brain barrier. Additionally, DA was found not only to be resistant to but also inhibitory toward glyoxalase I, the primary initiator of detoxification of amyloid-promoting reactive dicarbonyl species that are generated naturally in large amounts by neuronal tissue. In light of the chronic exposure of industry workers to DA, this study raises the troubling possibility of long-term neurological toxicity mediated by DA.
Journal information: Chemical Research in Toxicology
Provided by American Chemical Society