A protein thwarts developmental abnormalities by preventing removal of critical chemical marks from embryonic DNA
When a mammalian egg gets fertilized, it essentially undergoes a genomic ‘reset’ that transforms it into an embryonic cell capable of developing into the full spectrum of adult tissues. Daniel Messerschmidt and co-workers at the A*STAR Institute of Medical Biology have now identified the protein TRIM28 as a key player in this reprogramming process.
Genetic activity is not exclusively determined by sequences of nucleotides, but also depends on ‘epigenetic marks’ — chemical modifications, such as the addition of methyl groups to DNA, that can dramatically affect gene expression. After fertilization, many parental genes are stripped of their methyl groups (a process called demethylation), but certain maternally- or paternally-inherited copies of specific genomic loci are ‘imprinted’ and retain their original methylation pattern.
TRIM28 normally contributes to DNA methylation, which makes it seem a likely contributor to establish imprinting patterns, but Messerschmidt and his team have determined that this is not the case. “To our surprise, TRIM28 is not required to establish imprints, as we might have predicted, but instead helps maintain these features after fertilization,” says Messerschmidt.
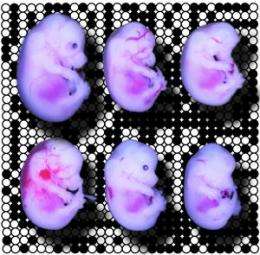
In the earliest stages of development, gene expression depends entirely on proteins synthesized from maternal messenger RNA (mRNA) within the oocyte. Therefore, the researchers investigated the early function of this gene with embryos generated by using sperm from normal male mice to fertilize oocytes that lack the Trim28 gene. Messerschmidt and co-workers observed a staggering variety of defects in the resulting embryos (see image), none of which successfully developed to term.
Closer analysis revealed unexpected demethylation of numerous loci that are normally imprinted, indicating that TRIM28 typically insulates these regions against epigenetic modification. Some loci were more commonly affected than others, but overall the effects were highly heterogeneous among littermates. “We were surprised by the extreme variability,” says Messerschmidt, “as all the embryos were genetically identical and should therefore display the same defects.”
Interestingly, subsequent expression of the intact paternal Trim28 gene was insufficient to rescue these embryos later in development, suggesting that damage arising from early abnormalities accumulates over the course of development. “Once a defect has occurred and imprinting is lost at a locus, it cannot be recovered, impacting on later embryonic stages,” says Messerschmidt. By exploring how TRIM28 protects its target genes and attempting to identify other, non-imprinting-related functions for this protein, Messerschmidt and his team hope to gain insights that might ultimately prove valuable in predicting — or possibly even preventing — human birth defects.
More information: Messerschmidt, D. M. et al. Trim28 is required for epigenetic stability during mouse oocyte to embryo transition. Science 335, 1499–1502 (2012). dx.doi.org/10.1126/science.1216154