New regulatory mechanism discovered in cell system for eliminating unneeded proteins
A faulty gene linked to a rare blood vessel disorder has led investigators to discover a mechanism involved in determining the fate of possibly thousands of proteins working inside cells.
St. Jude Children's Research Hospital scientists directed the study, which provides insight into one of the body's most important regulatory systems, the ubiquitin system. Cells use it to get rid of unneeded proteins. Problems in this system have been tied to cancers, infections and other diseases. The work appears in today's print edition of the journal Molecular Cell.
Researchers demonstrated how a protein named Glomulin binds to a key component of the regulatory system. Investigators showed not only where Glomulin binds but also how binding shuts down a biochemical cascade that tags unnecessary proteins for dismantling.
The findings highlight a potential new approach for future studies aimed at developing treatments for glomuvenous malformations, which are associated with mutations in the gene for making the Glomulin protein. The malformations, which are often present at birth, result in veins that cause discolored raised skin lumps that are sometimes painful and disfiguring.
"The findings suggest it might someday be possible to treat disorders resulting from defects in proteins that shut off the ubiquitin system by finding an alternative mechanism for turning the pathway off. The way Glomulin works represents a new way of controlling one of the most important regulatory systems at work in cells. We believe Glomulin may represent the tip of the iceberg. There could be many proteins that work in this fashion," said Brenda Schulman, Ph.D., a member of the St. Jude Department of Structural Biology and a Howard Hughes Medical Institute investigator. She is the study's senior and corresponding author.
Cells rely on this ubiquitin system to keep proteins in balance. As part of the ubiquitination process, a small protein is passed from one enzyme to the next like a baton in a relay race until it reaches a protein complex known as the cullin RING ligase. There, the small protein is transferred to a waiting target protein, marking it for destruction.
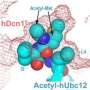
In this study, researchers showed that Glomulin disrupts ubiquitination by "glomming" onto and masking the spot on the cullin RING ligase where the tagging process is completed. The binding site is on the RBX1 protein, a component of the cullin RING ligase. While Glomulin derives its name from the disease, researchers showed the name also aptly describes Glomulin's molecular function.
The study builds on earlier work from the laboratory of James DeCaprio, M.D., of the Dana-Farber Cancer Institute, Boston. DeCaprio, a coauthor of this study, and his colleagues reported that Glomulin regulates cullin RING ligases by binding to the RBX1 protein. They also reported that Glomulin does not bind to the related RBX2 protein.
In this latest study, St. Jude scientists used X-ray crystallography to determine the structures involved in Glomulin's interaction with RBX1 and another component of the cullin RING ligase. "This was one of those 'ah-ha' moments," Schulman said. "Seeing the structure told us almost immediately how Glomulin disrupts the ubiquitination process."
Additional tests showed that Glomulin binds tightly to the surface of the RBX1 protein. The binding prevents the enzyme carrying the kiss-of-death protein tag from delivering its cargo. The structure also suggests how Glomulin mutations associated with glomuvenous malformations prevent the protein from binding to RBX1.
Researchers showed that Glomulin disrupts ubiquitination by binding to the same spot on the cullin RING ligase where the enzyme carrying the ubiquitin protein must bind to complete the process. The location is on the RBX1 protein, which is part of the larger protein complex.
"RBX1 regulates potentially thousands of different proteins within the cell. Glomulin represents a new way of regulating this entire class of RBX1 associated proteins," said David Duda, Ph.D., a scientist in Schulman's laboratory and the study's first author. Glomulin is the first protein made by a cell shown to block a site like the one on RBX1.