New immune therapy treats brain tumors in mice
Using an artificial protein that stimulates the body's natural immune system to fight cancer, a research team at Duke Medicine has engineered a lethal weapon that kills brain tumors in mice while sparing other tissue. If it can be shown to work in humans, it would overcome a major obstacle that has hampered the effectiveness of immune-based therapies.
The protein is manufactured with two arms – one that exclusively binds to tumor cells and another that snags the body's fighter T-cells, spurring an attack on the tumor. In six out of eight mice with brain tumors, the treatment resulted in cures, according to findings published Dec. 17, 2012, in the Proceedings of the National Academy of Sciences.
"This work represents a revival of a somewhat old concept that targeting cancer with tumor-specific antigens may well be the most effective way to treat cancer without toxicity," said senior author John H. Sampson, M.D., PhD, a neurosurgeon at The Preston Robert Tisch Brain Tumor Center at Duke. "But there have been problems with that approach, especially for brain tumors. Our therapeutic agent is exciting, because it acts like Velcro to bind T-cells to tumor cells and induces them to kill without any negative effects on surrounding normal tissues."
Sampson and colleagues focused on the immune approach in brain tumors, which are notoriously difficult to treat. Despite surgery, radiation and chemotherapy, glioblastomas are universally fatal, with a median survival of 15 months.
Immunotherapies, in which the body's B-cells and T-cells are triggered to attack tumors, have shown promise in treating brain and other cancers, but have been problematic in clinical use. Treatments have been difficult to administer at therapeutic doses, or have spurred side effects in which the immune system also attacks healthy tissue and organs.
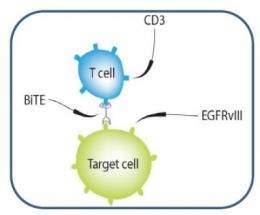
Working to overcome those pitfalls, the Duke-led researchers designed a kind of connector - an artificial protein called a bispecific T-cell engager, or BiTE – that tethers the tumor to its killer. Their newly engineered protein includes fractions of two separate antibodies, one that recruits and engages the body's fighter T-cells and one that expressly homes in on an antigen known as EGFRvIII, which only occurs in cancers.
Once connected via the new bispecific antibody, the T-cells recognize the tumor as an invader, and mount an attack. Normal tissue, which does not carry the tumor antigen, is left unscathed.
"One of the major advantages is that this therapy can be given intravenously, crossing the blood-brain barrier," said lead author Bryan Choi, a dual M.D-PhD candidate at Duke. "When we gave the therapy systemically to the mice, it successfully localized to the tumors, treating even bulky and invasive tumors in the central nervous system."
The team also developed an antidote to other current immune-targeting therapies that have a toxic effect, enhancing their safety profiles and bolstering their effectiveness.
"Additional studies will concentrate on whether these findings can be replicated in human trials, and whether the treatment is affected by the use of current therapies such as radiation and chemotherapy," Sampson said.















