Enzyme CaM kinase II relaxes muscle cells: Researchers find overactive enzyme in failing hearts
A certain enzyme, the CaM kinase II, keeps the cardiac muscle flexible. By transferring phosphate groups to the giant protein titin, it relaxes the muscle cells. This is reported by researchers led by Prof. Dr. Wolfgang Linke of the Institute of Physiology at the Ruhr Universität in the journal Circulation Research.
In failing hearts, which don't pump enough blood around the body, the scientists found an overly active CaM kinase II. "The phosphorylation of titin could be a new starting point for the treatment of heart failure," Prof. Linke speculates.
Titin phosphorylation determines the mechanical tension of the muscle cell
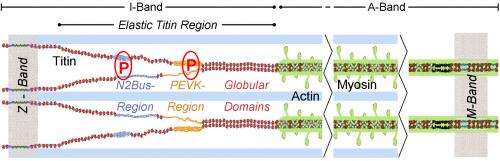
Titin is the largest protein in the human body, and it acts like a spring which tenses or relaxes the muscle cell. The attachment of phosphate groups to specific titin sites - known as phosphorylation - relaxes the cell. It was already known that the calcium/calmodulin-dependent kinase II, CaM kinase II for short, phosphorylates several proteins in heart cells. Whether it also targets the spring protein titin, has now been examined by the researchers in Bochum.
CaM-Kinase II phosphorylates the giant protein titin
For the study, the researchers used heart cells of "normal" mice, mice that have no CaM kinase II, and mice that produce more CaM kinase II than usual. In cells without the enzyme, titin phosphorylation was reduced by more than 50 percent compared to the normal state. In cells with excess enzyme, however, titin phosphorylation was twice as strong as in normal cells. The CaM kinase II is therefore crucial for the attachment of phosphate groups to the giant protein titin. Linke's team identified two regions within the flexible segment of the titin molecule which are phosphorylated by the enzyme, namely the PEVK and N2Bus region. These sites contain several amino acids of the type serine and threonine, which have changed little in the course of evolution.
The work of the CaM kinase II determines cell stiffness
In further analyses, the research team also showed that a lack or an excess of CaM kinase II affected the stiffness of the muscle cells. Cells without the enzyme were stiffer, cells with the enzyme more flexible. If they added CaM kinase II to cells that were not able to produce the enzyme themselves, these relaxed. In failing human hearts, the team found increased activity of CaM kinase II in comparison with healthy hearts, and thus excessive phosphorylation in the PEVK and N2Bus titin regions. "This seems to alter the mechanical properties of the human heart muscle", says Wolfgang Linke.
More information: Hamdani, N. et al. (2012): Crucial role for Ca2+/Calmodulin-dependent Protein Kinase-II in regulating diastolic stress of normal and failing hearts via Titin phosphorylation, Circulation Research, DOI: 10.1161/CIRCRESAHA.111.300105