Many cancer patients suffer from a dramatic loss of fat and muscle mass. This extreme wasting, or cachexia, is often the actual cause of death in cancer patients. Heidelberg scientists have now discovered in mice that tumors stimulate the production of a key gene switch in the liver. Activity of this switch lowers blood fat levels so that the animals lose weight. This finding may lead to approaches to slow down this fatal loss of body mass.
Cachexia or wasting is a condition affecting up to 70 percent of cancer patients, depending on the type of cancer. It is characterized by a dramatic loss of body weight that is independent of food intake. Cachexia is seen particularly often and most pronounced in patients suffering from cancers of the digestive tract and the lungs. They may lose up to 80 percent of body fat and skeletal muscle. Muscle loss leads to weakness and immobility of patients and poorer response to treatment. An estimated 20 percent of cancer deaths are considered to be a direct consequence of cachexia, with failure of the respiratory muscles as a frequent cause of death.
"Doctors used to believe that cancer re-programs metabolism in such a way that all energy goes into tumor growth," says Prof. Dr. Stephan Herzig, who heads a joint research department of the German Cancer Research Center (DKFZ), Heidelberg University and Heidelberg University Hospital. However, by now researchers presume that cachexia is the body's response to various harmful stimuli originating directly from the growing tumor. In his endeavor to find the causes of cachexia, Stephan Herzig, an expert in metabolism, took a closer look at the liver as the control center of metabolism for the first time. "Cachexia patients frequently have an inflamed fatty liver – this was a major clue for this organ being involved."
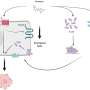
The researchers discovered that cancerous mice have extremely low lipid (blood fat) levels – meaning that their bodies lack the most important energy source. However, they accumulate fat in the liver. The low lipid levels in the diseased animals are due to their liver releasing only very little VLDL (very low density lipoprotein). This lipoprotein is the vehicle that transports fats in the blood. Moreover, the genes for all major steps of lipogenesis are blocked in the livers of cancerous mice.
"This is a clear indication of a central gene switch in the liver driving cachexia", says Stephan Herzig. The researcher therefore specifically searched for differences in protein switches regulating gene activity and hence hepatic energy metabolism in cancerous and healthy mice. Herzig's team found significant differences in a poorly studied gene switch called TSC22D4, which is found in larger amounts in cancerous mice than in healthy control mice.
Herzig's team demonstrated the key role of TSC22D4 in the onset of cachexia. The researchers specifically silenced the switch in the animals' livers. The organ subsequently went back to producing enough VLDL to make lipid levels in the cancerous animals rise. In addition, the genes involved in lipogenesis got boosted again.
"Our results prove, for the first time, that dramatic loss of body mass may be controlled by the liver," says Stephan Herzig. "We also know by now that TSC22D4 has exactly the same effect in human hepatic cells. There is evidence suggesting that this gene switch can be controlled via specific metabolic products and that we might thus be able to slow down the fatal wasting process. However, this approach has not yet been proven experimentally. This is what we will investigate next."
More information: Allan Jones, Kilian Friedrich, Maria Rohm, Michaela Schäfer, Carolyn Algire, Philipp Kulozik, Oksana Seibert, Karin Müller-Decker, Tjeerd Sijmonsma, Daniela Strzoda, Carsten Sticht, Norbert Gretz, Geesje M. Dallinga-Thie, Barbara Leuchs, Manfred Kögl, Wolfgang Stremmel, Mauricio Berriel Diaz and Stephan Herzig: Transforming growth factor-beta1 Stimulated Clone-22 D4 is a molecular output of hepatic wasting metabolism, EMBO Molecular Medicine, 2012
Journal information: EMBO Molecular Medicine
Provided by Helmholtz Association of German Research Centres