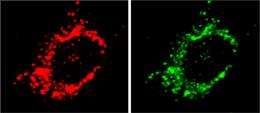
The fragmentation of mitochondria (red) in muscle cells is promoted by Mul1 protein (green). Credit: 2012 Elsevier
Muscle withering can occur as part of the progression of many diseases, including cancer and muscular dystrophy, as well as during the normal aging process. Cellular organelles known as mitochondria provide energy for muscle contraction, and their fragmentation within muscle cells can lead to muscle wasting. Now, a team of researchers led by Ravi Kambadur at the A*STAR Singapore Institute for Clinical Sciences has identified a key role for mitochondrial E3 ubiquitin protein ligase 1 (Mul1) in mitochondrial fragmentation. Such fragmentation occurs in response to stimuli that cause muscle loss.
Starvation and the use of anti-inflammatory steroid drugs can induce muscle wasting in animals. In cell culture experiments, the researchers found that these same stimuli could cause mitochondrial dysfunction and fragmentation in muscle cells. More specifically, these stimuli increased the expression of the Mul1 protein. In turn, this led to a decrease in the levels of a protein called Mfn2, resulting in the mitochondria breaking apart. Interestingly, normal levels of Mfn2 expression led mitochondria to fuse with one another.
When the researchers overexpressed Mul1 in muscle cells, instead of fusing with other mitochondria, these organelles merged with a cellular compartment called the lysosome in which proteins and organelles are degraded. Exposing muscle cells to starvation or steroids also led to fusion between mitochondria and lysosomes. However, Kambadur and co-workers found that they could block this fusion by silencing the expression of Mul1, effectively preventing degradation of the mitochondria.
Kambadur and his team observed that, in keeping with its known role of marking proteins to be degraded with ubiquitin tags, Mul1 binds and adds ubiquitin groups to Mfn2, leading to Mfn2 degradation. They then showed that once degraded, Mfn2 can no longer drive mitochondrial fusion, which tips the balance such that the mitochondria begin to fragment.
When Mul1 was overexpressed in the muscle of mice, the researchers observed a drop in muscle weight. Upon starvation, mice normally experience muscle loss, but Kambadur and co-workers were able to block this wasting by preventing the increased expression of Mul1 that is normally triggered by starvation. These findings indicate that Mul1 is required for the mitochondrial fragmentation and muscle loss caused by stimuli that normally break down muscle.
Next, the team will focus on determining whether Mul1 also induces muscle wasting in human muscle cells under various nutrition stress conditions. "If it does," says Kambadur, "the major clinical application, I believe, would be treatment of anorexia that normally leads to heavy muscle wasting."
More information: Lokireddy, S., Wijesoma, I. W., Teng, S., Bonala, S., Gluckman, P. D. et al. The ubiquitin ligase Mul1 induces mitophagy in skeletal muscle in response to muscle-wasting stimuli. Cell Metabolism 16, 613–624 (2012). www.cell.com/cell-metabolism/abstract/S1550-4131%2812%2900405-6
Journal information: Cell Metabolism