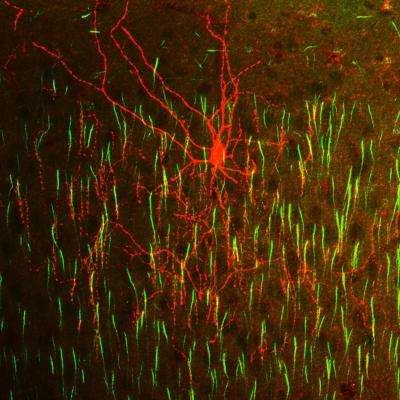
A chandelier cell (red) innervates multiple axon initial segments (green) in mouse neocortex. Credit: Hiroki Taniguchi
(Medical Xpress)—Neuroscientists have sought to explain diseases like schizophrenia and autism in terms of abnormal development of cortical microcircuitry. Before leaping to high level functional conclusions from low level anatomy, it may be a good idea to ask what low level functions can even be inferred from structure. A new perspective paper in Science titled, " Neuronal Birth to Cortical Circuitry," takes stock of new techniques for tracing the development of some of the more colorful players on the cerebral roster. Chief among them are the enigmatic "chandelier cells" that can usurp control over the output of nearly all the pyramidal cells in their vicinity. Chandelier synapses are able to outcompete all comers to command the most highly coveted real estate in all the cortex—the axon initial segment. The ability to electrically patch onto targeted subtypes of developing cortical cells, while imaging the effects of their activity on the surrounding network now offers the opportunity to directly map microscale anatomy to function. Where sterile electron micrographs (EM) previously only hinted at the dynamic storm continually unfolding at every synapse, researchers might now begin to expand the limited notion of static cortical "circuits" under the enlightened understanding that development processes in the brain might slow down, but they never really end.
Chandelier cells are distinguished from other GABA-ergic interneurons by their unique "cartridge" synaptic arrays, and fast-spiking activity. Although attempts have been made to correlate patterns of spike activity with observables like mitochondrial size and distribution, definitive conclusions form EM images are impossible. In some cases, like the giant "Calyx of Held" synapse found in the auditory system, structure-function links are more obvious. The idea that such a large and excrescenced synapse is required to support sustained, fast and reliable signal transmission for sound source localization in the auditory system, is easier to digest. To prove ideas like these, researchers can now use techniques that spatially and temporally label these unique components for study.
To visualize chandelier cells in particular, an inducible site-specific Cre recombinase can be used for genetic fate mapping of the homeobox gene Nkx. Chandelier cells are the latest-born interneurons that are derived from the Nkx-expressing progenitor family, and Nkx is required for their development. By crossing a particular kind of Cre-mice (Nkx2.1CreERT2) with other mice expressing a fluorescent fusion protein, it is possible to create an animal where only cells that originate from a certain region and time—like chandelier cells—can be targeted. These mice can be induced to express the indicator protein upon injection of an appropriate activating molecule, in this case tamoxifen. The "ER" in Nkx2.1CreERT2 stands for estrogen receptor, and the "T" presumably for tamoxifen.
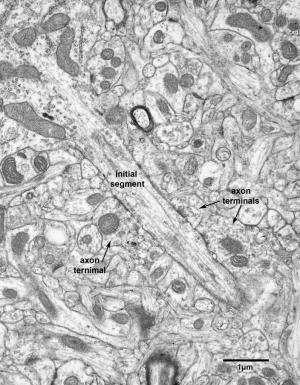
The axon initial segment of a pyramidal neuron in layer 4 of the visual cortex of a 6 year old monkey. The axon initial segment is forming symmetric, inhibitory synapses, with three axon terminals. These axon terminals are from a chandelier cell. Credit: Dr. Alan Peters, Boston University School of Medicine
The Science perspective also describes other cases where specific neuron subtypes, like those that contain somatostatin (SST), can be targeted. Within the SST interneuron subgroup, layer 3 neuron subtypes specifically contacted pyramidal cell dendrites, while layer 4 subtypes synapsed onto fast-spiking interneurons. At some point, asserting increasingly fine-grained cerebral axioms, like the sometimes fuzzy imposition of layer specificity, can create a situation where there description, together with the exceptions, becomes so long that the actual full connectome might be seen as the more compact descriptor. Furthermore, many of these kinds of axioms continually change in development.
For example, it is well established that the ultimate effects of the transmitter GABA flip-flop during maturation between having an excitatory and an inhibitory influence. In chandelier cells, these effects have been observed physiologically. Researchers have suggested that such transmitters must do more than just toggle membrane potential up and down. Synapses at the initial segment are uniquely poised to control other aspects of neural function like, for example, axonal transport, or even initial reverse-propagating spikes. Evidence that reverse spiking in pyramidal cells during sleep has a critical role in cortical microcircuits was recently discovered by Douglass Field and his group at NIH.
One antidote to the unbounded proliferation of increasingly specific molecular axiom is to instead search for underlying principle. The focus of the Science perspective is on circuit development, with a critical emphasis placed on the successive timing of waves of migrating neurons. The idea that the different species of neurons, and other cells in the cortex, must occupy a particular niche that becomes available at the time they first at their destination is powerful. For example, one might ask, why do pyramidal cells adopted their particular morphology and deep-layer position?
It is difficult to get neurons to develop the same circuit structure in culture as they do in vivo. As with different species of tree, genetics obviously plays a huge role in the structures neurons adopt. One might be tempted to ask whether the fine-scale particulars of their branch patterns any more meaningful in reality than those of trees? In parts of the visual system like the retina there is ample evidence that it can and does. For the cortex, the concept is more difficult to support.
For the pyramidal cells, a deep layer makes eminent sense if your large axon is going to project down to the white matter anyway, myelin notwithstanding. The long apical dendrite, and even the side-projecting dendrite structure then logically follow. Later arriving neurons then pluck down and branch according to where they can access nutrient ultimately from the capillaries that is has metabolized through the synaptic mill into the form they most desire. Through evolution the trend has been to expand pyramidal cell scope in terms of size and synapse number. The additional synaptic excitement garnered by these super-pyramidals has generally been tempered by shifting the typically cell-body specific inhibition further afield. This has often been accompanied by inflating the inhibitory synaptic influence into full-blown interneurons, replete with the flexibility therein.
These broadly painted strokes do not circumvent the need to obtain the full molecular picture, but rather compliment it. The migration of neurons in development sorts them, in effect, like proteins or DNA running down a gel. Along the way, molecular adhesion in the local environment, and internal stabilization of the underlying cytoskeleton are among the critical factors. Pacing advance on the molecular and structural end, with incite on the functional end through new techniques like those mentioned here will be critical for neuroscience going forward.
More information: The Spatial and Temporal Origin of Chandelier Cells in Mouse Neocortex, Science 4 January 2013: Vol. 339 no. 6115 pp. 70-74 DOI: 10.1126/science.1227622
ABSTRACT
Diverse γ-aminobutyric acid–releasing interneurons regulate the functional organization of cortical circuits and derive from multiple embryonic sources. It remains unclear to what extent embryonic origin influences interneuron specification and cortical integration because of difficulties in tracking defined cell types. Here, we followed the developmental trajectory of chandelier cells (ChCs), the most distinct interneurons that innervate the axon initial segment of pyramidal neurons and control action potential initiation. ChCs mainly derive from the ventral germinal zone of the lateral ventricle during late gestation and require the homeodomain protein Nkx2.1 for their specification. They migrate with stereotyped routes and schedule and achieve specific laminar distribution in the cortex. The developmental specification of this bona fide interneuron type likely contributes to the assembly of a cortical circuit motif.
Perspective: www.sciencemag.org/content/340/6136/1058.full
Journal information: Science
© 2013 Medical Xpress