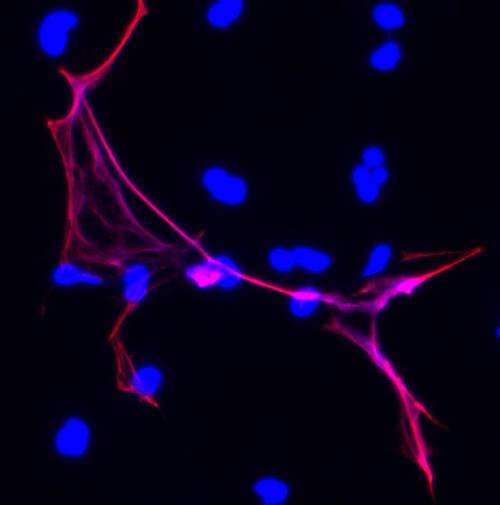
Shown in blue is chromatin -- the condensed form of DNA that the cell remodels to form chromosomes. The PAD4 enzyme -- histones modified by PAD4 shown in fuchsia -- decondenses chromatin by loosening up the interaction between DNA and special proteins called histones. This process helps to form both a bacteria-killing NET -- which is comprised of infection-combatting white blood cells called neutrophils -- and the fluffy, scattered ball that comprises a blood clot. Credit: Wang lab, Penn State University. Originally published in the Journal of Cell Biology.
(Medical Xpress)—A gene associated with both protection against bacterial infection and excessive blood clotting could offer new insights into treatment strategies for deep-vein thrombosis—the formation of a harmful clot in a deep vein. The gene produces an enzyme that, if inhibited via a specific drug therapy, could offer hope to patients prone to deep-vein clots, such as those that sometimes form in the legs during lengthy airplane flights or during recuperation after major surgery. The research, which was led by Yanming Wang, a Penn State University associate professor of biochemistry and molecular biology, and Denisa Wagner, senior author with decades of research on thrombosis at the Boston Children's Hospital and the Harvard University Medical School, will be published in in the Online Early Edition of the journal Proceedings of the National Academy of Sciences during the week ending 10 May 2013.
The team's new findings are an extension of previous research by Wang and other scientists. In earlier studies, Wang and his colleagues had revealed that a gene in mice called Pad4 (peptidylarginine deiminase 4) produces an enzyme that plays an important role in protecting the body from infection. The researchers discovered that cells with a functioning PAD4 enzyme are able to build around themselves a protective, bacteria-killing web that is dubbed a NET (neutrophil extracellular trap).
Now, in their new research, team members have studied the PAD4 enzyme's role in clotting. Wang explained that, as a part of its NET-producing duties, PAD4 regulates the formation of chromatin—the condensed form of DNA that the cell remodels to form chromosomes. "PAD4 decondenses chromatin by loosening up the interaction between DNA and special proteins called histones. The resulting chromatin threads then combine with protein fibers, blood platelets, and other materials to become, not only the bacteria-killing NET, but also the fluffy, scattered ball that comprises a blood clot." Wang added that, in some individuals, blood clots tend to form within deep veins. These clots can then travel to the heart, causing cardiac arrest, or to the lungs, causing breathing problems.
In one of their experiments, team members compared mice with a normally functioning Pad4 gene to mice with a defective gene. They found that, when veins were constricted, genetically normal mice—those able to produce the PAD4 enzyme—formed clots as expected. However, genetically mutated mice—those unable to produce the enzyme—did not form clots normally. In fact, the scientists noted a two-fold difference in clot formation between genetically normal and genetically abnormal mice at six hours after the procedure. After 48 hours, the difference had reached 10-fold. "We noted some clotting activity in these genetically abnormal mice, but the clots were not as bulky and were not maintained over time," Wang said. "Clearly, the PAD4 enzyme plays a critical role in the formation of a blood clot, as well as in the formation of a bacteria-fighting NET."
In another experiment, the research team transferred infection-combatting white blood cells—called neutrophils—from normal mice to genetically mutated mice. First author Kim Martinod, a graduate student in the Immunology Graduate Program at the Harvard University Medical School, found that, in response to vein constriction, these "rescued" mice now could function normally, forming clots as efficiently as mice with a functioning Pad4 gene, demonstrating that the Pad4 gene did produce a functioning PAD4 enzyme in these white blood cells to regulate blood clotting.
"PAD4, which is also called PADI4 in humans, is a necessary enzyme involved in multiple disorders," Wang explained. "On the one hand, it plays an integral part in the body's defense system, as we showed in earlier work: It is necessary in the production of the protective, bacteria-killing NET. On the other hand, our earlier work also showed that this enzyme acts to silence tumor-suppressor genes. Now, in our new research, we are starting to see that its overactivity also may be part of the reason that some individuals suffer from deep-vein clotting." Wang added that patients prone to deep-vein thrombosis might benefit from drugs that target the PAD4 enzyme. "In future research, specific drug therapies could be developed and tested with the goal of targeting this enzyme," Wang said. "If we could find a way to dial back the enzyme's clot-forming effects, we might be able to offer new hope to patients suffering from clotting disorders and deep-vein thrombosis."
Journal information: Journal of Cell Biology , Proceedings of the National Academy of Sciences
Provided by Pennsylvania State University