Spine function improves following cell replacement therapy with fetal human stem cells
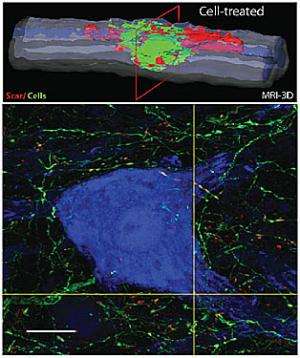
Human foetal stem cell grafts improve both motor and sensory functions in rats suffering from a spinal cord injury, according to research published this week in BioMed Central's open access journal Stem Cell Research and Therapy. This cell replacement therapy also improves the structural integrity of the spine, providing a functional relay through the injury site. The research gives hope for the treatment of spinal cord injuries in humans.
Grafting human neural stem cells into the spine is a promising approach to promote the recovery of function after spinal injury. Sebastian van Gorp, from the University of California San Diego, and team's work looks specifically at the effect of intraspinal grafting of human foetal spinal cord-derived neural stem cells on the recovery of neurological function in a rats with acute lumbar compression injuries.
A total of 42 three month-old female Sprague-Dawley rats, with spinal compression injuries, were allocated to one of three groups. The rats in the first group received a spinal injection with the stem cells, those in the second group received a placebo injection, while those in the third group received no injection.
Treatment effectiveness was assessed by a combination of measures, including motor and sensory function tests, presence of muscle spasticity and rigidity which causes stiffness and limits residual movement. The team also evaluated of how well the grafted cells had integrated into the rodents' spines.
Gorp and colleagues found that, compared to rats who received either the placebo injection or no injection, those who received the stem cell grafts showed a progressive and significant improvement in gait/paw placement, reduced muscle spasticity as well as improved sensitivity to both mechanical and thermal stimuli. In addition to these behavioural benefits, the researchers observed long-term improvements in the structural integrity of previously injured spinal cord segments.
The authors say: "Importantly, spinal cavity formation and muscle spasticity are frequently observed in human patients with high-speed, high-impact induced spinal cord injuries. Our findings demonstrate that human foetal spinal cord-derived neural stem cells, with an already established favorable clinical safety profile, represent a potential cell candidate for cell replacement therapy in patients with traumatic spinal injuries."
More information: Amelioration of motor/sensory dysfunction and spasticity in a rat model of acute lumbar spinal cord injury by human neural stem cell transplantation, Sebastiaan van Gorp, Marjolein Leerink, Osamu Kakinohana, Oleksandr Platoshyn, Camila Santucci, Jan Galik, Elbert A Joosten, Marian Hruska-Plochan, Danielle Goldberg, Silvia Marsala, Karl Johe, Joseph D Ciacci and Martin Marsala, Stem Cell Research & Therapy 2013 4:57. stemcellres.com/content/4/3/57/abstract