Engineering tissue to rebuild damaged bones and organs
From the chimera in Greek mythology to the sphinx in ancient Egypt, humans have imagined making creatures from pieces of different organisms for millennia.
Tissue engineering, the innovative field that uses engineering principles to develop biological substitutes for cells or even major organs, is just the latest version, says Gordana Vunjak-Novakovic, the Mikati Foundation Professor of Biomedical Engineering and Medical Sciences.
"From American Indians 3,000 years ago to the Renaissance in Italy, noses were repaired using human tissue parts," she says. "Today, we are combining major advances in stem cell biology, bioengineering and clinical medicine to grow biological substitutes for our failing tissues."
As director of Columbia's Laboratory for Stem Cells and Tissue Engineering, Vunjak-Novakovic's lab grows all sorts of human tissues, from beating heart patches to bone grafts that can match a patient's original jaw.
"We look at how things happen in the body, both during normal development and pathological function, and reproduce these conditions using engineering principles," says Vunjak-Novakovic.
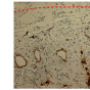
Jaw reconstruction is closest to being used in clinical trials. The process starts by obtaining CT scans of bone that needs reconstruction. These are converted into three-dimensional images and used to create a scaffold in the exact shape and size of the defect.
Learn more about Prof. Vunjak-Novakovic's research (Credit: Columbia News Video Team)
The same images are used to make a chamber into which the scaffold is tightly fit. Then the scaffold is infused with mesenchymal stem cells—the kind that can differentiate into different varieties of cell types—obtained from the patient's fat and fed with a medium containing nutrients, growth factors and oxygen. "We condition the bone for three weeks and fit it into the defect," she explains. "You would actually grow a piece of bone that would totally resemble the bone you are replacing or regenerating."
This "grow your own bone" approach could offer alternatives to current methods of using inorganic materials such as titanium, which cannot adapt to the body. "If the patient is young, the titanium wouldn't grow with the person or change as we age," says Vunjak-Novakovic. "We are trying to replace these materials with living human bone grown in the lab." This approach was reported in 2009 in the Proceedings of the National Academy of Sciences and recently validated in large animal studies.
Tissue cells form specific structures critical for their function. Cardiac muscle cells, for example, organize themselves in parallel formations, enabling the heart to contract and pump blood through the 100,000 miles of blood vessels in the body. Cells without specific spatial organization may never become fully functional if they do not replicate in the proper structure, Vunjak-Novakovic said. Her lab creates large quantities of tissues, to better study their structure and investigate drug therapies.
George Eng, a research assistant in the MD/PhD program, recently devised a method to make tissues that can be assembled like Legos. He created a template and differently shaped hydrogel capsules—a material with flexibility similar to natural tissue—that fit together in a lock-and-key manner. This new tissue-creating technique lets researchers construct unique and controlled cell patterns that allow precise studies of cell function in response to many different environments. This approach was reported in the March 4, 2013 issue of the Proceedings of the National Academy of Sciences.
Life in the lab started early for Vunjak-Novakovic. She grew up in Belgrade, Serbia, in the former Yugoslavia, where her father, a chemical engineer, arranged for her to spend time in labs during the summers. She received her Ph.D. in chemical engineering from the University of Belgrade and traveled to the United States in 1986 with her husband and son to study at the Massachusetts Institute of Technology on a Fulbright fellowship. There, a meeting with MIT Professor Robert Langer, considered a pioneer of tissue engineering, was pivotal. "When he explained he was working on tissue engineering, I said 'tissue what?' but it immediately became a lifelong professional niche for me," she recalls.
She collaborated with Langer for years but became a visiting professor at MIT in February 1993, when the republics of the former Yugoslavia were already at war. "My poor country was falling to pieces so we kept extending our stay," Vunjak-Novakovic recalls. Later that year she joined the MIT faculty full time, and got a green card. She became a naturalized citizen in 2000 and arrived at Columbia in 2005.
Vunjak-Novakovic sees today as a golden age for the field of tissue engineering. "I expect that during our lifetime we will be able to leave a mark and help people live longer, better lives."