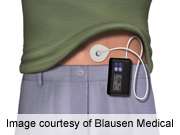
For patients with type 1 diabetes and documented nocturnal hypoglycemia, insulin-pump therapy with a threshold-suspend feature reduces nocturnal hypoglycemia over a three-month period, according to a study published online June 22 in the New England Journal of Medicine to coincide with presentation at the annual meeting of the American Diabetes Association, held from June 21 to 25 in Chicago.
(HealthDay)—For patients with type 1 diabetes and documented nocturnal hypoglycemia, insulin-pump therapy with a threshold-suspend feature reduces nocturnal hypoglycemia over a three-month period, according to a study published online June 22 in the New England Journal of Medicine to coincide with presentation at the annual meeting of the American Diabetes Association, held from June 21 to 25 in Chicago.
Richard M. Bergenstal, M.D., from the International Diabetes Center at Park Nicollet in Minneapolis, and colleagues compared sensor-augmented insulin-pump therapy with and without a threshold-suspend feature in 247 patients with type 1 diabetes and nocturnal hypoglycemia. Participants were randomized to receive sensor-augmented insulin-pump therapy with the threshold-suspend feature (121 patients) or standard sensor-augmented insulin-pump therapy (126 patients) for three months.
The researchers observed similar changes in glycated hemoglobin values in both groups. For nocturnal hypoglycemic events, the mean area under the curve was 37.5 percent lower in the threshold-suspend versus the control group. In the threshold-suspend group, nocturnal hypoglycemic events occurred 31.8 percent less frequently than in the control group. In the threshold-suspend group, the percentages of nocturnal sensor glucose values of less than 50 mg/dL, 50 to less than 60 mg/dL, and 60 to less than 70 mg/dL were significantly lower. Four patients experienced a severe hypoglycemic event, all in the control group.
"This study showed that over a three-month period the use of sensor-augmented insulin-pump therapy with the threshold-suspend feature reduced nocturnal hypoglycemia, without increasing glycated hemoglobin values," the authors write.
The study was funded by Medtronic MiniMed, manufacturer of the pumps used in the study.
More information:
Abstract
Full Text
More Information
Journal information: New England Journal of Medicine
Health News Copyright © 2013 HealthDay. All rights reserved.