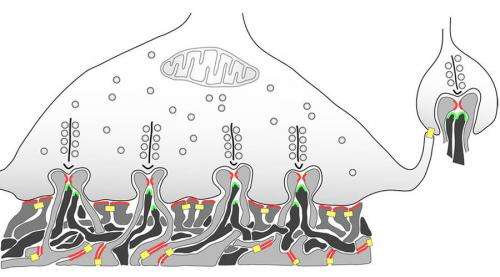
Multiple Synaptic Ribbons for sustained vesicle release. Credit: brain.mpg.de
(Medical Xpress)—Neurophysiologist like to think of neurons as communicating with spikes. If that were the whole story, it might be possible to imagine spike codes which could then be used to estimate the flow of information, and perhaps energy, in the brain. The reality as most in the field know, is that neurons do their bidding with transmitter-charged vesicles. The principles of vesicle operation, and by implication any codes that might be involved, are entirely different from those of spikes. While much of neuroscience has concerned itself with the interaction of these two phenomena, they have yet to be satisfactorily reconciled. A recent review appearing in Trends in Neuroscience suggests that sensory systems might offer the best clues towards divining the form of what we might call, an electric potential to vesicle fusion "transfer function." In particular, the researchers examine the ribbon synapses that tend to be found wherever fast, sustained and reliable transmission may be required. By making the case that spikes and graded potentials have complementary roles in transducing sensory information into vesicle fusion, they seek to better define the costs of synaptic transmission.
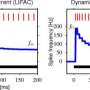
Typically, graded potentials can track a continuous stimulus with much greater temporal resolution than spikes because there are far fewer ions needed to carry the signal. There is no significant refractory period involved, and far less energy is used in restorative pumping or scavenging. They can also voltage-match a greater dynamic range in a stimulus than a fixed amplitude spike might hope to temporally encode. The downside is that graded potentials, like analog signals, are in principle less immune to noise, and they do not travel well. That is why spikes are generally chosen for longer distance transmission even though they are much more energy intensive.
In the retina, analog to digital conversion for transmission to the brain occurs at more than one level. The bipolar cells have a substantial investment in ribbon synapses which presumably support precision graded-level transduction. However, it has emerged that spiking, at least in the form of multi-ion "spikelets," may also be a critical part of their repertoire, and therefore the two modes are not exclusive. One concern in a situation like this is that if the cell switches often between graded and spiking modes, the spikes, which might be expected to cause relatively prodigious release, would tend to get expensive. Vertebrate retinas also make extensive use of synaptic ribbons within the far more numerous photoreceptor cells.
One of the most abused and gerrymandered terms in all neuroscience might be said to be the RRP—the rapidly releasable pool. This largely theoretical construct derives from attempts to reconcile experimental data with electron micrograph images of synapse. It would presumably correspond to those fully-filled vesicles which would be expected to be mobilized and fused by the action of the next spike to come down the pike. A common estimate for the RRP tends to hover around 20 vesicles. In reality this number depends on many things, not least being the spike history, but also whether those spikes were the real deal, or just the little burps of subthreshold stimuli in the more compact sensory cells. Beyond that, the synapse can only be expected to perform to its potential if it has successfully attracted and retained sufficient mitochondrial backers to supply ATP and restore cationic order.
Unfortunately it is not enough just to consider a single synapse in isolation to derive its hypothetical transfer function. A synapse is far from being the only sheep in the flock, and it must ultimately answer to the soma if it becomes too liberal. Although attempts have been made to estimate the energy budget of a single neuron from imaging studies, the accuracy of the method is very limited. Efforts to do theoretical ATP neuroaccounting will invariably come up short due to the many unseen effciencies availible in actual metabolic pathways. Long story short, if every synapse of an axon is ready at guard to fire at every action potential, not only is one of the most powerful, information-enriching and homeostatic mechanisms available to neurons compromised, but the neuron itself might likely be expired.
On the dendritic side, the situation is not so much an immediate energy problem, but rather an information bottleneck, or loss, that would occur if every dendritic synapse was potentially active and responding. An onslaught of backpropagating or reverse-directed axonal spikes might present an additional load to these postsynaptic sites, but there is no vesicle-related overhead in this case. Clearly there is much speculation involved here. It is however, a necessary extrapolation when attempting to properly account for trade-offs between spikes and potentials, or between high performance synaptic ribbons and traditional synapses. The efforts of the researchers in this most recent paper to better define the functional role of synaptic structure is a much needed and refreshing approach.
More information: Spikes and ribbon synapses in early vision, Trends in Neurosciences, 22 May 2013. DOI: 10.1016/j.tins.2013.04.006
Summary
Image processing begins in the retina, where neurons respond with graded voltage changes that must be converted into spikes. This conversion from 'analog' to 'digital' coding is a fundamental transformation carried out by the visual system, but the mechanisms are still not well understood. Recent work demonstrates that, in vertebrates, graded-to-spiking conversion of the visual signal begins in the axonal system of bipolar cells (BCs), which transmit visual information through ribbon-type synapses specialized for responding to graded voltage signals. Here, we explore the evidence for and against the idea that ribbon synapses also transmit digital information. We then discuss the potential costs and benefits of digitization at different stages of visual pathways in vertebrates and invertebrates.
© 2013 Medical Xpress