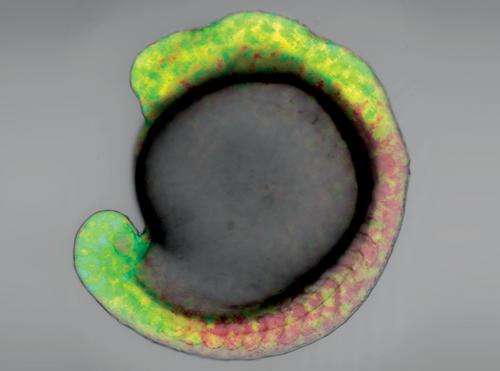
The retinoic acid concentration gradient in a zebrafish embryo expressing GEPRA-B, 16 hours after fertilization. Credit: 2013 Satoshi Shimozono, RIKEN Brain Science Institute
The formation of a 'body plan' during embryonic development is driven by the distribution of signaling molecules called morphogens in the embryo, determining front from back and left from right. Atsushi Miyawaki, Satoshi Shimozono and colleagues from the Laboratory for Cell Function Dynamics at the RIKEN Brain Science Institute, have now developed fluorescent probes that allow the distribution of the morphogen retinoic acid (RA) to be visualized in a living embryo for the first time, providing new insight into the important role that morphogens play in patterning the body during embryonic development1.
Morphogens are produced in specific areas of the embryo and diffuse through the developing organism. The resulting concentration gradient guides the development of the embryo. Retinoic acid is thought to play a critical role in the formation of the front-to-back axis of the brain, but its concentration gradient has never been observed directly and its very existence remains controversial.
The research team genetically engineered probes consisting of a segment of the RA receptor fused to cyan and yellow fluorescent proteins, and then expressed these proteins in zebrafish embryos. These genetically encoded probes for RA—known as GEPRAs—change conformation when they bind to RA, resulting in a change in the fluorescence they produce.
Key to the technique was the use of two probes with different binding affinities for RA—one binding very strongly and the other relatively weakly such that they each fluoresce in response to different concentrations of RA. This enabled the researchers to visualize the changes in concentration of RA within a living zebrafish embryo by fluorescence imaging (Fig. 1).
The imaging studies confirmed that RA is distributed along a concentration gradient in the hindbrain region during the early stages of embryonic development. "RA is synthesized in the trunk and degraded in the head and tail," says Shimozono. "Therefore, two gradients should form, with the highest concentration at the trunk, and declining towards either end."
RA is known to interact with another morphogen called fibroblast growth factor 8 (FGF8), which was previously believed to be closely associated with the formation of the RA concentration gradient. However, Miyawaki's team found that the RA concentration was unaffected by the blocking of FGF8 during hindbrain development, suggesting that FGF8 is not needed for gradient formation.
"We will now use GEPRAs to investigate how RA diffuses in the body at the cellular and the molecular level," says Shimozono.
More information: Shimozono, S., et al. Visualization of an endogenous retinoic acid gradient across embryonic development. Nature 496, 363–366 (2013). dx.doi.org/10.1038/nature12037
Journal information: Nature
Provided by RIKEN