A bad alliance: Rare immune cells promote food-induced allergic inflammation in the esophagus
Food is an integral part of life; but, for some, it can be harmful. Allergic inflammation caused by inappropriate immune responses to some types of food has become a major public health issue. Over the past ten years, the prevalence of food allergies has increased by nearly 20 percent, affecting an estimated six million people in the U.S.
Eosinophilic esophagitis (EoE) is a food allergy-associated disease that affects children and adults and is caused by inflammation in response to such trigger foods as eggs, nuts, milk, wheat, and soy. Inflammation of the esophagus, as seen in EoE patients, can eventually lead to debilitating esophageal dysfunction, causing difficulty in swallowing, esophageal fibrosis, and food impaction. However, current treatment options for EoE, including adherence to strict diets, are non-specific and disruptive to patients' lifestyle.
Until recently, the mechanisms underlying the development of EoE were unclear, but a new study from the Perelman School of Medicine at the University of Pennsylvania and The Children's Hospital of Philadelphia (CHOP) shows that a type of rare immune cell and specific reactions to allergenic foods team up – in a bad way – to cause EoE. However, this association does point to new ways to possibly treat inflammation associated with EoE.
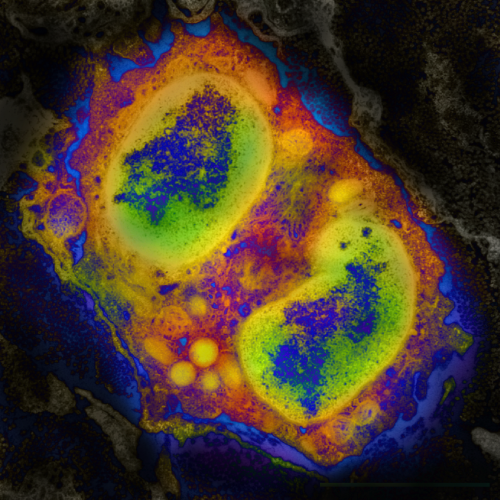
The presence of large populations of immune cells in the esophagus of human patients with EoE suggests that the immune system might contribute to the pathogenesis of this disease. In earlier work, researchers from CHOP, along with collaborators at Cincinnati Children's Hospital, found that genetic mutations in the gene that encodes for thymic stromal lymphopoietin (TSLP), a protein that is produced by epithelial cells that line the esophagus and directs the activities of various types of immune cells, are highly associated with EoE in children. These results suggested that TSLP played an important role in the development of this disease, but how this factor contributed to esophageal inflammation in response to food was unknown.
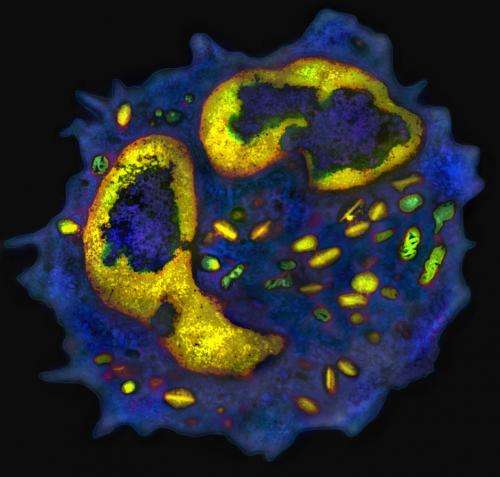
Now, David Artis, PhD, associate professor of Microbiology at Penn, two postdoctoral researchers in the Artis lab, Mario Noti, PhD and Elia Tait Wojno, PhD, and colleagues, have identified one mechanism by which TSLP might contribute to the development of EoE. They describe their work this week online ahead of print in Nature Medicine.
Using a mouse model of EoE, Artis's group found that sensitization to egg and peanut protein, in association with increased levels of TSLP, led to the mobilization of a rare type of immune cell called basophils. In healthy people, these cells comprise less than 1 percent of the total immune cells in the body. In EoE, however, these rare cells pack a punch – when mice with EoE were treated with therapeutic reagents that limited TSLP and basophil responses to food allergens, esophageal inflammation in these animals improved dramatically.
"The use of this new mouse model has revealed that TSLP production, and the resulting basophil responses, may be critical in promoting EoE in response to exposure to allergy-triggering foods," says Noti.
Supporting experiments in mouse models, the research team also found exaggerated TSLP and basophil responses in the esophageal biopsy tissues of pediatric and adult patients with EoE. What's more, pediatric EoE patients with a genetic mutation in the TSLP gene were more likely to have increased basophil responses in their blood compared to EoE patients that lacked this mutation.
"The identification of TSLP and basophil responses in the esophagus and peripheral blood of human patients with EoE supports our mouse model studies and indicates that these factors may play a key role in EoE in patients," says Tait Wojno.
The findings from both mouse and human studies suggest that TSLP and basophils may promote the development of inflammation in the esophagus in response to foods that trigger an allergic response in some individuals, or that TSLP and basophils could contribute to the persistence of inflammation. These factors could potentially be targeted using novel therapeutics to treat EoE in patients, say the researchers.
"Although more research is required, these studies suggest that we may be able to target TSLP and basophils to treat esophageal inflammation associated with EoE," adds Artis.
More information: Nature Medicine DOI: 10.1038/nm.3281