Muscle health depends on sugar superstructure
For many inherited diseases, such as cystic fibrosis or Huntington disease, the disease-causing genetic mutation damages or removes a protein that has an essential role in the body. This protein defect is the root cause of the disease symptoms.
However, for a group of muscular dystrophies known collectively as congenital muscular dystrophies (CMDs), the sequence of the protein that is central to normal function is typically unaffected. Instead, the defects lie in processing proteins—ones that are responsible for modifying the central protein by adding sugar chains (glycans). Either loss of the glycans or disruption of their structure is sufficient to cause muscle disease.
In a new study, published online Aug. 8 in the journal Science, a University of Iowa team led by Kevin Campbell, Ph.D., has pinpointed not just one, but three proteins that are required for constructing a key, early section of a critical sugar chain. Mutations affecting any one of these three proteins can cause CMD disease in humans.
The central protein in CMDs is dystroglycan (DG). "It looks like at least 10 to 15 genes encode proteins that contribute to the glycan superstructure that makes DG effective," says Campbell, professor and head of molecular physiology and biophysics at the UI Carver College of Medicine, and a Howard Hughes Medical Institute investigator. "Our goal is to figure out the whole pathway by which the glycan structure is built, since defects in any of the proteins can potentially lead to disease. Knowing which genes are involved is expected to help us develop clinical tests for these dystrophies, and also ways to screen for potential therapeutic agents."
Normally, DG is modified with a unique sugar chain that acts like glue, allowing DG to attach to other proteins and, by doing so, to reinforce cell membranes in many tissues—including muscle and brain. DG does not function properly without this sugar modification, and glitches in the construction of the glycan cause the progressive muscle dysfunction and the brain abnormalities that characterize many forms of muscular dystrophy.
Almost a dozen genetic mutations are now known to cause DG-related CMDs, which include Fukuyama Congenital Muscular Dystrophy, Walker-Warburg Syndrome, Muscle-Eye-Brain disease, and certain types of limb-girdle muscular dystrophy. All of these mutations affect proteins (enzymes) that are responsible for building DG's unique sugar chain.
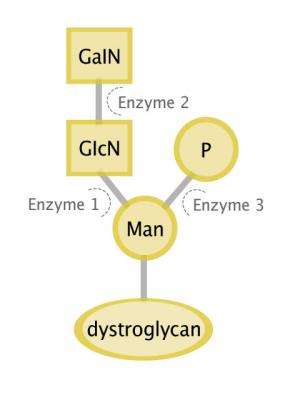
The new study assigns a role to three of these causative mutations, showing that the three affected enzymes act sequentially to build an early section of DG's critical glycan. When any of these proteins are mutated, the sugar chain is not constructed correctly and the DG protein loses its function. The first author of the study was Takako Yoshida-Moriguchi, Ph.D., a UI research assistant professor in Campbell's lab
The three enzymes connect a series of sugars together to form the glycan; like stringing beads together to make a necklace. The starting point of the chain is a mannose sugar, which is attached to the backbone of the DG protein. The first enzyme analyzed in the study, called GTDC2, links a glucosamine (GlcNAc) sugar to this starting mannose. The second enzyme, B3GALNT2, then adds a galactosamine sugar to the GlcNAc. Only when this disaccharide is complete can a third enzyme—an unusual type of kinase called SGK196—add a phosphate group to the mannose at the beginning of the chain.
Earlier work from Campbell's lab has shown that this phosphate link is required for other enzymes to build the final section of the sugar chain—the part that actually allows dystroglycan to do its job.
"What I find really exciting is that even with the whole genome having been described, we are still finding novel enzymes that carry out functions we didn't know about even two or three years ago," Campbell says.
Identifying these enzymes and understanding their functions may eventually provide leads for developing therapies to treat CMD and other muscle diseases, Campbell adds.
More information: "SGK196 is a Glycosylation-Specific O-Mannose Kinase Required for Dystroglycan Function" Science, 2013.