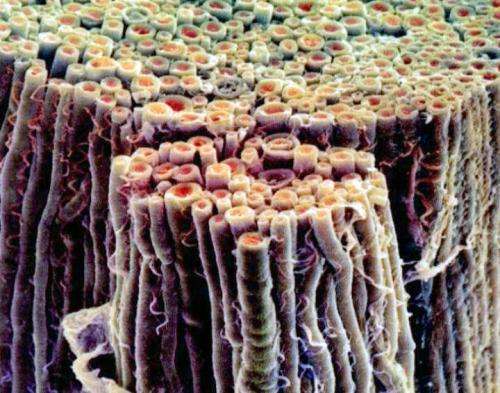
Section of myelinated nerve segments. Credit: education.vetmed.vt.edu
(Medical Xpress)—When Descartes turned his critical eye to the nervous system, he reasoned that the nerves must transduce hydraulic power to control the musculature. In the circulatory system, blood is pushed comparatively slowly through the aorta, perhaps around 0.3 meters per second. Superimposed on that flow, however, is an arterial pulse wave which propagates much faster, both through the blood and the walls of the vessel. For compliant and healthy vessels that speed might be around 10 meters per second, while for more hardened arteries, it could be 15 or higher. Modern day electrophysiologists have since replaced the hydraulic model with the idea that nerves really only transmit information—electrical information no less. Yet when looking at the power supply to the leg, for example, it is still hard to ignore the fact that the main femoral artery, at a diameter scarcely a half of an inch, looks rather meager next to the "information-supplying" sciatic nerve, which may actually be more like three-quarters of an inch. A conflux of ideas from a variety of disciplines has recently led to a critical re-emergence of the more mechanical side of the nervous system. To that point, two German scientists have just published a paper in the journal, Medical Hypotheses, where they suggest that the pulse wave is the main event in nervous conduction, while the electrical show is mere epiphenomenon.
We recently discussed the increasingly popular idea that action potentials may actually be soliton waves which propagate in the membranes of axons as phase transitions with minimal loss in energy. Convincing biologists that these subtle creatures could exist in the chaotic and varied conditions inside neurons has been a challenge. However, it is harder to argue against the fact that any kind of electrochemical spike based on the rapid influx of ions will be accompanied by a significant pressure pulse. The idea that the German researchers have supported, is that these as the pressure pulses naturally decay in the viscoelastic medium of the nerve, they are refreshed by ionic input at the nodes between myelinated axon segments, or continuously in unmyelinated axons.
If you have ever been absent-minded enough to grab a live wire, or even brush up strongly against one, the sensation is unforgettable. It is not such a stretch to acknowledge that when you slam your funny bone, or more precisely the Ulnar nerve (largest unprotected nerve in your body), the resultant vibe and decay feels almost identical to a real electrical assault. Similarly, the so-called "stingers" that run down the limbs after a sharp blow to the head are familiar to most footballers, and can give one quite a shock. Unfortunately these (albeit very simplistic) macroscopic intuitions don't hold up so well when extended to the microscopic domain. Granted, when the electrochemical mechanisms that are assumed to underlie nervous conduction are looked at in detail, it becomes more difficult to disentangle the mechanical from the electric. However, as the authors observe, at some point, an attentive electrophysiologist must ask his or herself, "why are so many ion channels mechanosensitive" ?
Original view of nerves from Descartes. Credit: cns.nyu.edu
One unexpected finding of the patch clamp recording technique was that the dilation of the membrane caused by local tension leads to considerable increase in transmembrane ion flow. Impulse waves causing short extensions in the membrane can directly induce opening and closing of both voltage and ligand gated channels. The idea that the pore in these channels is a rigid tube isolated from larger membrane events is difficult to support in this context. According to the authors, it is quite likely that common mechanoreceptor devices, like the pressure- or vibration-sensitive Vater-Pacinian corpuscles of the skin, conduct signals to initiate high-speed polysynaptic muscle reflex circuits without any classical intermediary electrical conversion.
The exact conduction velocity of mechanical impulses in nerve fibers remains unknown. It is estimated that under physiologic conditions, an unamplified axoplasmic pressure pulse would decay over roughly 1 mm due to viscosity, depending on the distensibility of the axon wall. When compared to the theoretical case of an absolutely rigid wall, a typical myelin sheath may be rigid enough to support pulse speeds up to one-fifth of the estimated maximum. That speed is not to shabby when compared with some rough estimates from previous authors, which put the maximum pulse velocity under an indistensible membrane somewhere upwards of 1500 meters per second. Suddenly, the quicker than life eyeblink response, or speed of the tenderfoot stepping on a sharp shard, become a little more comprehensible.
The theory as it stands is incomplete and needs to be adapted for specific cases with real biology in mind. In different animals, and in different regions of their brains, conduction in neurons goes by different names. For example, in the cerebellum, the unmyelinated parallel fibers pack to extreme densities in a regular crystalline lattice whose reason to be defies physiologic explanation to this day. Just as we currently have no good explanation for how signals could be properly isolated in nerve bundles where seemingly random nodes of Ranvier overlap in extent and influence, it is hard to imagine parallel fibers could maintain their electrochemical, or even mechanical, autonomy within this geometry.
The pressure wave theory wields considerable predictive power when it comes to explaining some of the unique synaptic specializations found throughout the brain. When considered only from an electrochemical point of view, the huge structural synaptic investments, like those found at the neuromuscular junction (NMJ), can hardly be imagined to be driven by local, and weak, current or field effects. One might need look no further than simple-to-recreate Chaldni patterns set up in two dimensions on the surface of a taunt drum, to make the imaginative leap to a three dimensional system, with multiple vibrating players, where more extreme patterns might easily be set up to provide authorship to repeatable complex structure. For the NMJ in particular, the case has been made that at the end-plate, the comparatively enormous efflux of acetylcholine to the deeply-guttered cleft, and propagation of excitation through the transverse tubule system, are all components of a continuous mechanical amplifier.
The apparent ease with which evolving organisms manage to cobble together all manner of sensitive hearing devices becomes infinitely more explicable once we see that nature has apparently been doing this kind of thing all alone inside of neurons. The amplification and transduction through liquid channels, of barely noticeable vibrations against a background of thermal noise much greater in magnitude, is in this light, no evolutionary stumble-upon, but rather the bread and butter of neural systems, and perhaps many aspects of life in general.
More information: Impulses and pressure waves cause excitement and conduction in the nervous system, Medical Hypotheses, Article in Press, www.medical-hypotheses.com/art … (13)00373-3/abstract
Abstract
It is general accepted, that nerval excitement and conduction is caused by voltage changes.
However, the influx of fluid into an elastical tube releases impulses or pressure waves. Therefore an influx of ion currents, respectively fluid motions into the elastic neuronal cells and fibres also induce impulses. This motion of charge carriers are measured by voltage devices as oscillations or action potentials, but the voltage changes may be an epiphenomenon of the (mechanical) impulses.
Impulse waves can have a high speed. As stiffer or inelastic a tube wall, the greater is the speed of the impulse. Myelin sheaths cause a significant stiffening of the nerve fibre wall and myelinated fibres have a conduction velocity up to 120m/s. The influx of fluid at the nodes of Ranvier intensifies periodically the impulse wave in the nerve fibres.
The authors suggest that also the muscle end-plate acts as a conductor of axonal impulses to the inner of the muscle fibres and that the exocytosis of acetylcholine into the synaptic cleft may be an amplifier of the axonal impulse. It is discussed that intracellular actin filaments may also influence motions at the neuronal membrane.
Many sensory nerve cells are excited due to exogenous or endogenous mechanical impulses. It may plausible that such impulses are conducted directly to the sensory nerve cell bodies in the dorsal root ganglia without the transformation in electric energy. Excitation conduction happens without noteworthy energy consumption because the flow of ion currents through the membranes takes place equivalent to the concentration gradient.
Impulse waves cause short extensions of the lipid membranes of the cell- and fibres walls and therefore they can induce opening and closing of the included ion channels. This mechanism acts to "voltage gated" and "ligand-gated" channels likewise.
The concept of neuronal impulses can be helpful to the understanding of other points of neurophysiology or neuronal diseases. This includes e.g., the brain concussion and pathohistological findings in Alzheimer dementia.
To verify the concept of (mechanical) impulses in the nervous system it is necessary to carry out biophysical or mechanical investigations in very small dimensions and the authors hope to give for this a sufficient stimulus.
Journal information: Medical Hypotheses
© 2013 Medical Xpress