Radiotherapy in girls and the risk of breast cancer later in life
Exposing young women and girls under the age of 20 to ionizing radiation can substantially raise the risk of their developing breast cancer later in life. Scientists may now know why. A collaborative study, in which Berkeley Lab researchers played a pivotal role, points to increased stem cell self-renewal and subsequent mammary stem cell enrichment as the culprits. Breasts enriched with mammary stem cells as a result of ionizing irradiation during puberty show a later-in-life propensity for developing ER negative tumors - cells that do not have the estrogen receptor. Estrogen receptors - proteins activated by the estrogen hormone - are critical to the normal development of the breast and other female sexual characteristics during puberty.
"Our results are in agreement with epidemiology studies showing that radiation-induced human breast cancers are more likely to be ER negative than are spontaneous breast cancers," says Sylvain Costes, a biophysicist with Lawrence Berkeley National Laboratory (Berkeley Lab). "This is important because ER negative breast cancers are less differentiated, more aggressive, and often have a poor prognosis compared to the other breast cancer subtypes."
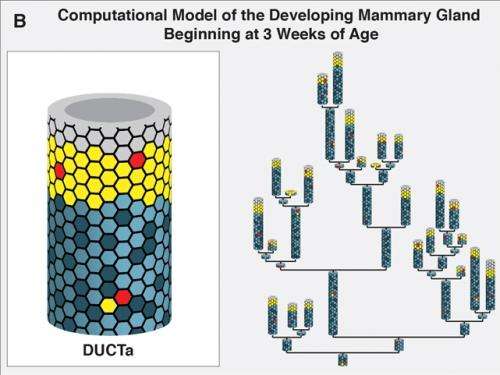
Costes and Jonathan Tang, also with Berkeley Lab, were part of a collaboration led by Mary Helen Barcellos-Hoff, formerly with Berkeley Lab and now at the New York University School of Medicine, that investigated the so-called "window of susceptibility" known to exist between radiation treatments at puberty and breast cancer risk in later adulthood. The key to their success were two mammary lineage agent-based models (ABMs) they developed in which a system is modeled as a collection of autonomous decision-making entities called agents. One ABM simulated the effects of radiation on the mammary gland during either the developmental stages or during adulthood. The other simulated the growth dynamics of human mammary epithelial cells in culture after irradiation.
"Our mammary gland ABM consisted of millions of agents, with each agent representing either a mammary stem cell, a progenitor cell or a differentiated cell in the breast," says Tang. "We ran thousands of simulations on Berkeley Lab's Lawrencium supercomputer during which each agent continually assessed its situation and made decisions on the basis of a set of rules that correspond to known or hypothesized biological properties of mammary cells. The advantage of this approach is that it allows us to view the global consequences to the system that emerge over time from our assumptions about the individual agents. To our knowledge, our mammary gland model is the first multi-scale model of the development of full glands starting from the onset of puberty all the way to adulthood."
Epidemiological studies have shown that girls under 20 given radiotherapy treatment for disorders such as Hodgkin's lymphoma run about the same risk of developing breast cancer in their 40s as women who were born with a BRCA gene mutation. From their study, Costes, Tang and their collaboration partners concluded that self-renewal of stem cells was the most likely responsible mechanism.

"Stem cell self-renewal was the only mechanism in the mammary gland model that led to predictions that were consistent with data from both our in vivo mouse work and our in vitro experiments with MCF10A, a human mammary epithelial cell line," Tang says. "Additionally, our model predicts that this mechanism would only generate more stem cells during puberty while the gland is developing and considerable cell proliferation is taking place."
Costes and Tang are now looking for genetic or phenotypic biomarkers that would identify young girls who are at the greatest breast cancer risk from radiation therapy. The results of their study with Barcellos-Hoff and her research group show that the links between ionizing radiation and breast cancer extend beyond DNA damage and mutations.
"Essentially, exposure of the breast to ionizing radiation generates an overall biochemical signal that tells the system something bad happened," Costes says. "If exposure takes place during puberty, this signal triggers a regenerative response leading to a larger pool of stem cells, thereby increasing the chance of developing aggressive ER negative breast cancers later in life."
















