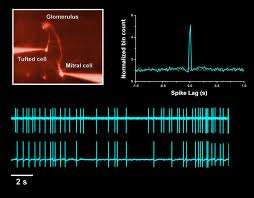
Spikes in the Olfactory Bulb. Credit: Monell.org
(Medical Xpress)—The olfactory system is a particular favorite among the many who study neural coding. One reason for this is that presentation of a single odorant to an otherwise featureless smellscape, at least in theory, provides an ideal and dimensionless event for the olfactory front end to code. There is ample evidence that within the olfactory bulb, odors are represented by spatial patterns of activity. There is also evidence that odors are captured by individual cells through spike timing, pattern, and their phase relative to the respiratory cycle. As expected, there is also much suggestion to the contrary for each of these cases. A new study done by researchers from Carnegie Mellon reports that the identity of an odor directly influences the amount of correlation, or linked firing, in the spikes of the output cells of the bulb. In their recent paper published in PNAS, they show that this correlation originates primarily from the act of sniffing itself, with significant contributions also arising from the local circuit connections (within a few cell diameters) in the bulb, while the odor itself contributes only a small portion of the correlation.
The output cells of the bulb, the Mitral and Tufted cells (MT cells) studied here, send their axons to various higher centers in the brain, but ultimately, the olfactory cortex. M/T cells appear to fire with fair degree of regularity, even in the absence of odorants, in contrast to the cortical cells which have generally have lower spontaneous rates. What really turns on the M/T cells though, is breathing. Different MT populations have been shown to favor different phases of the respiratory cycle, but individual preferences can still change on dime. Decades ago, researchers performed various manipulations including cutting nerve tracts, blocking the nostrils, tracheotomy, and presenting odorants directly to the epithelium, to try to uncover the origins of the respiratory control. They found the peripheral airflow effects were a primary driver, but that there was also some influence from above so to speak, presumably through an efference copy of the output of the respiratory center. There was also found to be an intrinsic effect, where the bulb in effect "rings" at various pre-tuned fundamentals or overtones according to its size and connectivity.
Other studies have focused on more fine-grained origins of MT cell correlations, like for example, intrinsic channel dynamics within cells or the localization of gap junctional proteins on the apical dendrites of conspiring cells. One study showed that the electrical pore forming protein, connexion 36, was critical to maintaining correlations in MT activity. To try to get a better handle on these different kinds of correlations, I asked author Nathan Urban about the coupling between firing rate and inspiration seen in his studies. He mentioned that this was not explicitly probed but that it is likely that the influence is from periodic activation of sensory neurons during breathing. Before trying to get too particular here about attributing precise mechanism to sometimes imprecise or even weak correlations, we might step back for a moment ask in ignorant bliss, is it possible to say anything at all with certainty about codes and odorants just by looking at spike trains in olfaction?
The olfactory system is not so much a hard-wired telephone network where channels can be instantaneously specified and connected by number codes, and then used to transmit further codes. There is significant overgrowth and back-pruning in olfactory networks during development, and some ongoing cell replacement, but once etched, many of its larger features remain stable. The front-end receptor cells of the network express only one kind of sensor, but this sensor may respond to several different kinds of odorants. Not only that, but each odorant can potentially activate several kinds of receptors. The complication here is that we really don't know all that much about what features of odorants they are sensing, and how they sense it. That makes understanding what is to be coded a challenge.
What happens in the development of the olfactory system, is that a kind of labelled-line network spontaneously emerges that is composed of around one or two thousand discrete synaptic glomeruli. As these glomeruli self-organize, they accept projections only from receptor cells possessing the same odor sensor. It is the glomeruli then, might be said to be the real detectors of the system, essentially transforming "odorant space" into "detector space." While there is generally no clear indication that any significant odor sorting occurs in the nasal passages or epithelium, or that any topography like that found in the retina is evident for receptor cells, these possibilities should not be written off entirely.
The critical feature of the circuit remodeling in the developing bulb, is that like the other senses, it is built on the back of the spiking activity of neurons. Once the circuits stabilize, the neurons obviously don't just shut down, but rather they keep on pumping out synchronized spikes to their entire axonal tree and beyond. The key here for neural codes, is that even as many of the protein isoforms expressed in critical periods of development are taken out of service, spikes can not be considered completely independent of their direct effect on circuit structure and tone. Nor can a quiescent stimulus space be considered neutral to a detector cell.
The timescale on which the authors found significant correlations was in the range of 10-100ms. They note that when noise in the system is correlated, information transmission can be reduced because the noise does not average out. They mention the intriguing possibility that noise correlation levels are matched to population sizes, and suggest that the number of MT cells associated to a particular glomerulus (~25 in vertebrates) represents an optimal level where beyond that, information transmission would begin to saturate.
There is one major caveat in considering the olfactory system as a communication and signal conditioning network—the so-called noise on the channels, that which is measurable even in the absence of a signal, is in reality anything but. The buzz of nerves in development transitions through breathing amniotic fluid, to air without a hitch, all the while updating the brain about the condition and state not only of its peripheral sensors, but of central more generalized activating systems. Any code we define for the bulb should be considerate of the fact that while perceiving the alarming smell of smoke or methane may feel exceptional to us at the cognitive level, for the nose, it may just be a handful of atoms toggling receptor states whose larger condition and health needs to be reported in roll call among countless others. In this light any message can not stand out like the clear dots and dashes of Morse code above noise, but must surf in the ocean where every wave is considered potentially meaningful.
More information: Origins of correlated spiking in the mammalian olfactory bulb, PNAS, Published online before print September 30, 2013, DOI: 10.1073/pnas.1303830110
Abstract
Mitral/tufted (M/T) cells of the main olfactory bulb transmit odorant information to higher brain structures. The relative timing of action potentials across M/T cells has been proposed to encode this information and to be critical for the activation of downstream neurons. Using ensemble recordings from the mouse olfactory bulb in vivo, we measured how correlations between cells are shaped by stimulus (odor) identity, common respiratory drive, and other cells' activity. The shared respiration cycle is the largest source of correlated firing, but even after accounting for all observable factors a residual positive noise correlation was observed. Noise correlation was maximal on a ∼100-ms timescale and was seen only in cells separated by <200 µm. This correlation is explained primarily by common activity in groups of nearby cells. Thus, M/T-cell correlation principally reflects respiratory modulation and sparse, local network connectivity, with odor identity accounting for a minor component.
Journal information: Proceedings of the National Academy of Sciences
© 2013 Medical Xpress