Human stem cells used to elucidate mechanisms of beta-cell failure in diabetes
Scientists from the New York Stem Cell Foundation (NYSCF) Research Institute and Columbia University Medical Center (CUMC) have used stem cells created from the skin of patients with a rare form of diabetes—Wolfram syndrome—to elucidate an important biochemical pathway for beta-cell failure in diabetes. The findings by Linshan Shang and colleagues were published today in Diabetes.
Scientists from NYSCF produced induced pluripotent stem (iPS) cells from skin samples from individuals with a rare form of diabetes, Wolfram syndrome. They then derived insulin-producing cells (beta cells) from these iPS cells, creating a human diabetes model in vitro. Next, they showed that the beta cells failed to normally secrete insulin because of protein-folding—or endoplasmic reticulum (ER) —stress. They found that a chemical, 4-phenyl butyric acid, that relieves this stress, prevents the cells from failing, suggesting a potential target for clinical intervention.
"These cells represent an important mechanism that causes beta-cell failure in diabetes. This human iPS cell model represents a significant step forward in enabling the study of this debilitating disease and the development of new treatments," said Dieter Egli, PhD, principal investigator of the study, and Senior Research Fellow at NYSCF and NYSCF–Robertson Stem Cell Investigator.
Wolfram syndrome is a rare, often fatal genetic disorder characterized by the development of insulin-dependent diabetes, vision loss, and deafness. Since all forms of diabetes are ultimately the result of an inability of pancreatic beta cells to provide sufficient insulin in response to blood sugar concentrations, this Wolfram patient stem cell model enables analysis of a specific pathway leading to beta-cell failure in more prevalent forms of diabetes. It also enables the testing of strategies to restore beta-cell function that may be applicable to all types of diabetes.
"Utilizing stem cell technology, we were able to study a devastating condition to better understand what causes the diabetes symptoms as well as discover possible new drug targets," said Susan L. Solomon, Co-Founder and Chief Executive Officer of The New York Stem Cell Foundation.
"This report highlights again the utility of close examination of rare human disorders as a path to elucidating more common ones," said co-author Rudolph L. Leibel, MD, the Christopher J. Murphy Professor of Diabetes Research and co-director of the Naomi Berrie Diabetes Center at CUMC. "Our ability to create functional insulin-producing cells using stem cell techniques on skin cells from patients with Wolfram's syndrome has helped to uncover the role of ER stress in the pathogenesis of diabetes. The use of drugs that reduce such stress may prove useful in the prevention and treatment of diabetes."
Clinicians from the Naomi Berrie Diabetes Center recruited Wolfram syndrome patients to donate a skin sample. All Wolfram patients had childhood-onset diabetes requiring treatment with injected insulin, and all had vision loss. Additional cell lines were obtained from Coriell Institute for Medical Research. The researchers at NYSCF "reprogrammed," or reverted, the skin cells to an embryonic-like state to become iPS cells. An iPS cell line generated from a healthy individual was used as a normal control.
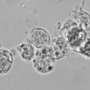
The researchers differentiated the iPS cells from the Wolfram subjects and the controls into beta cells, an intricate process that took several weeks. They implanted both Wolfram and control iPS cell-derived beta cells under the kidney capsule of immuno-compromised mice. Beta cells from the Wolfram subjects produced less insulin in the culture dish and secreted less insulin into the bloodstream of the mice when they were challenged with high blood-sugar levels.
A key finding was that these beta cells showed elevated markers of ER stress. Treatment with 4-phenyl butyric acid reduced the ER stress and increased the amount of insulin produced by the beta cells, thereby increasing the ability to secrete insulin in response to glucose.
Direct evidence in mice, as well as circumstantial evidence in humans with both type 1 and type 2 diabetes, highlights the role of the ER stress response mechanism in the survival of insulin-producing beta cells. The ER stress response mechanisms oppose both the stress of immune assault in type 1 diabetes and the metabolic stress of high blood glucose in both types of diabetes. When the ER stress response fails cell death occurs, potentially reducing the number of insulin-producing cells.